What Keeps A Candle Burning?
Candles have been used for light and ambiance for thousands of years, dating back to ancient China, Egypt, and Rome. Yet most of us don’t think twice about how they work their magic. At first glance a candle seems like a simple device, just a wick and wax. But there’s fascinating chemistry and physics happening inside that flickering flame. When you light a candle, you’re harnessing chemical energy and releasing it as heat and light. The candle transforms solid wax into gas through an intricate series of chemical reactions. With just the right materials and design, this controlled combustion can provide hours of glowing illumination. In this article, we’ll uncover the hidden science behind what keeps a candle burning.
The Wax
The wax used to make a candle is critical in keeping the candle burning. There are several types of waxes commonly used in candle making:
Paraffin wax is a petroleum byproduct made from crude oil refining. It is the most widely used candle wax due to its low cost and ability to hold fragrance. However, paraffin wax produces more soot as it burns and is not environmentally-friendly (source).
Beeswax is a natural wax made by honey bees. It has a lovely natural honey aroma and burns cleaner than paraffin wax. However, beeswax is more expensive and can be tricky to work with (source).
Soy wax is made from hydrogenated soybean oil. It’s a renewable and environmentally-friendly option that burns cleanly. The drawback is soy wax does not hold fragrance as well as paraffin (source).
Coconut wax comes from the pressed oil of coconut meat. It has excellent scent throw and produces minimal soot. However, coconut wax is soft and requires blending with other waxes (source).
The choice of wax impacts burn time, fragrance, and environmental footprint. Most modern candles use a blend of waxes to optimize performance.
The Wick
The wick is a crucial component in how a candle burns. The wick is generally made from braided strands of cotton, though other materials like wood, paper, and hemp can be used as well. The wick works by capillary action, drawing (“wicking”) the melted wax to the flame through the tiny gaps in the braided strands. This ensures a constant supply of fuel to the flame as the wax melts [1].
The number of braided strands and thickness of the wick determines how large the flame will be. Thicker wicks with more strands produce larger flames and are best for larger candles. Smaller wicks are needed for thinner candles to prevent excessive melting and sooting. Properly matching the wick size to the candle diameter is crucial for clean, efficient burning.
Materials like cotton and paper fiber char slowly as the candle burns, helping the wick curl and maintain an optimal burning shape. This prevents mushrooming at the tip which would block oxygen to the flame. The curled wick shape brings fresh fuel while leaving behind the charred portion in the molten wax pool.
Capillary Action
Capillary action is the ability of a liquid to flow against gravity in narrow spaces such as small tubes, pores, or cracks. It occurs when the adhesive intermolecular forces between the liquid and a solid surface are stronger than the cohesive intermolecular forces inside the liquid itself. This causes the liquid to be drawn upwards.
In a candle, capillary action causes the melted wax to be drawn up the wick. The wick is made up of many tiny fibers which act like narrow tubes. When the candle is lit, the heat melts the wax near the wick creating liquid wax. The adhesive forces between the liquid wax and the wick fibers are strong enough to pull the wax upwards. As the wax rises up the wick, it vaporizes and burns when it reaches the flame. This continuous capillary action of drawing up melted wax sustains the flame as long as there is still solid wax to melt.
For more information see: https://candles.org/candle-science/
Combustion
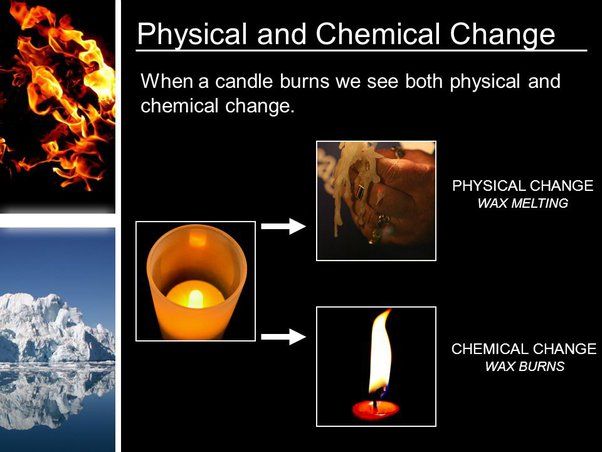
Combustion is a chemical reaction between a fuel and an oxidant, usually oxygen, that occurs at high temperatures and produces heat and light energy (https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/11%3A_Chemical_Reactions/11.06%3A_Combustion_Reactions). The fuel, such as wax or hydrocarbon compounds in a candle, reacts with oxygen to generate carbon dioxide, water vapor, heat, and light. Combustion cannot take place without oxygen, which is why candles cannot burn in an oxygen-free environment.
For combustion to occur, oxygen must be present in adequate concentrations. Oxygen makes up approximately 21% of Earth’s atmosphere. When a fuel source comes into contact with oxygen at high enough temperatures, an exothermic oxidation reaction occurs, providing the energy needed to keep the candle burning (https://www.britannica.com/science/combustion). The candle flame provides the high temperature needed to initiate and sustain the combustion reaction. The melted and vaporized wax fuel travels up the wick where it mixes with oxygen and ignites.
The combustion reaction converts the stored chemical energy in the hydrocarbon fuel to heat, light, and other products. As long as there is fuel available, oxygen present, and high enough temperatures, the combustion reaction is self-sustaining and the candle remains lit (https://www.chem.fsu.edu/chemlab/chm1020c/Lecture%207/01.php).
The Flame
The flame of a candle is divided into three distinct zones based on temperature and chemical reactions occurring. The innermost zone is the hottest and appears blue in color. This is the zone where wax vapors combine with oxygen to produce carbon dioxide and water vapor, releasing energy in the form of heat and light. According to Candle Flame, the blue region can reach up to 1400°C.
Surrounding the blue center is the yellow region, at a slightly cooler temperature between 1000-1200°C. This zone contains soot particles from the incomplete combustion of wax vapors. The yellow color comes from the glow of these incandescent carbon particles. Finally, the outermost zone appears orange or red, around 600-800°C. This region completes the combustion reaction started in the interior zones, as unburnt hydrocarbons mix with oxygen for a secondary burn.
The varying temperatures and chemical reactions occurring create the characteristic shape and color zones of a candle flame Candle flame – Introduction, Components, Structure with …. The hottest blue region provides the activation energy to sustain the overall combustion process.
Sooting & Mushrooming
Sooting and mushrooming refer to the black carbon buildup that can occur on candle wicks during burning. This carbon buildup causes the tip of the wick to form a mushroom-like shape.
According to https://support.candlescience.com/hc/en-us/articles/201668774-Why-does-my-candle-wick-have-a-black-mushroom-shape-after-burning, mushrooming happens when the flame consumes more wax than it can efficiently burn. This excess unburned wax solidifies into carbon that adheres to the wick, causing it to thicken and darken.
To prevent mushrooming, it’s important to use the properly sized wick for the diameter of the candle container. According to https://upcandledesign.com/blogs/mindful-artistry/candle-mushrooming-what-it-is-and-how-to-prevent-it, the most common cause of mushrooming is a wick that is too large for the candle’s diameter, which makes the flame too big and hot. Properly matching the wick size prevents mushrooming.
You can also prevent mushrooming by trimming the wick to 1⁄4” before each lighting, which removes any carbon buildup. Avoid drafts and fluctuating temperatures when burning candles. Use candle holders that are broad and sturdy to protect the flame.
Candleholder Design
The design of the candleholder plays an important role in keeping a candle burning. Proper candleholder design allows for heat dissipation and adequate airflow around the candle.
Tall, narrow holders that are open at the top are ideal for taper candles. This shape allows hot air to rise up and away from the flame, preventing the candle from overheating. Wide, shallow holders can trap heat and cause the candle to tunnel or form a deep melt pool, according to candle experts Honey Candle.
Candle plates, goblets or hurricanes can restrict airflow around a candle. It’s important to leave adequate headspace above the flame and not surround it fully with glass or another material. Votive candle holders work best when spaced apart to allow heat to dissipate between candles.
A properly designed holder can help manage heat and airflow to keep a candle burning evenly from top to bottom.
Troubleshooting
Candle making is a science and an art. Even experienced candle makers run into problems now and then. Here are some of the most common candle issues and how to fix them:
Sooting and Mushrooming
Sooting is when black smoke stains the wax around the wick. Mushrooming happens when the wick curls over instead of burning vertically. These issues are usually caused by the wick being too large for the wax pool. Switch to a smaller wick or trim the wick to 1⁄4″ before lighting to prevent excess soot and mushrooming (source).
Sinkholes
A sinkhole is a hole that forms in the top of the wax while the candle is burning. This happens when the wax near the wick melts faster than the wax on the outer edges. To fix minor sinkholes, use a heat gun to gently warm the wax so it levels back out. For major sinkholes, the wick may be too large or you may need to add more fragrance oil (source).
Wet Spots
Wet spots are spots on the candle wax that look wet, greasy, or sweaty. They are caused by the fragrance oil not fully incorporating with the wax. To prevent wet spots, make sure to stir the wax mixture for at least 2 minutes after adding fragrance. If wet spots form, try a lower fragrance oil concentration (source).
Conclusion: Repeating the Beautiful Cycle
As we have seen, a lit candle is an elegant example of science in motion. The capillary action draws wax up the wick, creating a small pool of molten wax that feeds the flame. The heat of the flame melts more wax to continue fueling the combustion reaction with oxygen in the air. This beautiful cycle repeats until the wax or wick is used up.
The various chemical and physical processes that enable a candle to burn – from the solid and liquid states of wax to vaporization to oxidation reactions – showcase how the seemingly simple chemistry happening all around us can create wondrous effects. Though a candle may seem mundane, reflecting on how it works can ignite our imagination and appreciation for the fundamental forces of nature at play.





