What Is The Basic Ingredient Of Making Soap?
Soap is a cleaning agent used for personal hygiene and laundering. The earliest recorded evidence of soap-like materials dates back to around 2800 BC in ancient Babylon [1]. Soap has played an essential role throughout history in preventing the spread of disease by eliminating dirt and microbes from the skin and other surfaces [2].
The key function of soap is its ability to emulsify – that is, to suspend oil/lipid dirt particles in water so they can be rinsed away. This emulsification occurs because soap molecules have a hydrophilic (water-loving) head and a hydrophobic (water-fearing) tail. The hydrophilic heads bond with water while the hydrophobic tails simultaneously bond with oil and grease on surfaces. This chemistry allows soap to act as a bridge between polar water and non-polar oils, allowing them to mix together and be washed away [3].
Fatty Acids
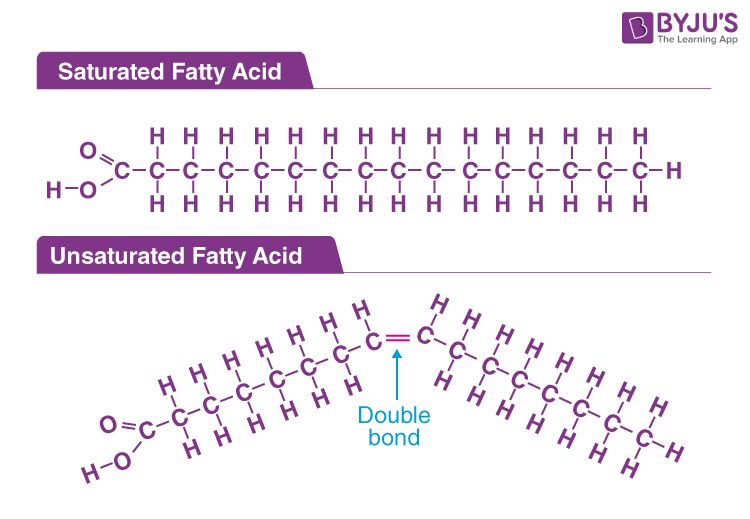
Fatty acids are the key component that make soap possible. Fatty acids are long chain monocarboxylic acids that have an alkyl group and a carboxyl group. The carboxyl group is what enables saponification, which is the chemical reaction between a fat or oil and a strong alkali to produce soap. Fatty acids vary in chain length and in the number of double bonds between carbon atoms. The properties of a fatty acid, including its melting point and solubility, are influenced by its molecular weight and degree of saturation. The most common fatty acids used in soap making are lauric, myristic, palmitic, stearic, oleic, linoleic, and ricinoleic acids.
Sources:
https://www.ultimateguidetosoap.com/post/why-every-beginner-should-learn-about-first
Saponification
Saponification is the chemical reaction that produces soap. It involves fatty acids reacting with an alkali, usually sodium hydroxide (lye) or potassium hydroxide. Here’s an overview of the saponification process:
Fatty acids are long hydrocarbon chains with a carboxylic acid group on one end. Common fatty acids in soap making include lauric acid, palmitic acid, oleic acid, and stearic acid. When these fatty acids encounter a strong base like lye, the acid group protonates, leaving behind the negatively charged carboxylate anion. This anion then associates with the positively charged sodium or potassium cation from the lye to form the salt we know as soap.
The overall reaction can be summarized as:
Fatty acid + Lye -> Soap + Glycerol
Where the fatty acid reacts with the hydroxide anion of the lye, forming the salt that is soap. The other product glycerol, is left over from the triglycerides found in fats and oils. The hydroxide breaks these triglycerides apart, forming three fatty acid chains and the glycerol backbone.
The saponification process is a exothermic reaction, meaning it generates heat as it proceeds. Cold process soapmaking relies on this heat to complete saponification at room temperature. The lye and oils are combined, then the reaction is kept at 95-100F over a period of several weeks until saponification runs to completion. This slow process results in a gentle, moisturizing soap.
Sources:
https://potagersoap.com/blogs/news/what-is-saponification
Lye
The most common alkali used in soapmaking is lye, also known as sodium hydroxide (NaOH). Lye is a caustic metallic base that produces an exothermic reaction when mixed with oils containing fatty acids. This chemical reaction, known as saponification, is the basic process behind soapmaking.
Lye is highly alkaline with a pH level of 13-14. When mixed with oils, the alkalinity of the lye interacts with the fatty acids in the oils to transform them into soap through saponification. Lye is soluble in water and is caustic, so proper safety precautions must be taken when handling it.
There are two main types of lye used in soapmaking:
- Sodium hydroxide (NaOH) – This is the most widely used lye for cold process and hot process soapmaking. It is commonly available in flake or bead form.
- Potassium hydroxide (KOH) – Also called potash lye, KOH makes a softer bar of soap. It is used in certain recipes, especially those containing high amounts of liquid oils.
The type and amount of lye needed depends on the oils used in a recipe. Online soap calculators help determine the exact amount of lye required to saponify the oils and create soap.
Overall, lye is a crucial base ingredient that initiates the chemical reaction to turn oils into soap through saponification. Understanding lye safety and using the proper amount in soap recipes allows for successful soapmaking.
Oils and Fats
The oils and fats used as sources of fatty acids in soapmaking generally fall into four main categories: olive oil, coconut oil, palm oil, and lard or tallow. Each oil contributes different properties to the final soap.
Olive oil is one of the most popular oils used in soapmaking. It contains oleic acid, which produces a mild, gentle soap that is moisturizing for the skin. Olive oil makes a creamy lather and produces a hard, long-lasting bar of soap. Extra virgin olive oil is sometimes used, but regular olive oil works well too.
Coconut oil contains lauric acid and myristic acid, which makes a hard soap that lathers easily. Coconut oil adds cleansing properties and bubbly lather to soap. However, too much coconut oil can be drying, so it is often combined with more moisturizing oils.
Palm oil, palm kernel oil, and palm shortening contain palmitic and stearic fatty acids. These oils produce a hard bar of soap that lathers well. However, palm oil production has negative environmental impacts, so some soap makers avoid it.
Animal fats like lard from pigs or tallow from cattle were commonly used in historical soapmaking. They produce a gentle, nourishing bar of soap that is compatible with skin. However, many modern soap makers use vegetable oils instead of animal fats.
Most soap recipes use a combination of oils and fats. A typical beginner recipe uses olive oil for moisturizing properties and coconut oil for bubbles. Adding small amounts of palm oil or lard will make the bar harder and longer-lasting. The art of soapmaking involves finding the right oil blend for the qualities you desire.
Lye Safety
When making soap from scratch, it’s crucial to follow safety precautions when handling lye, which is a caustic chemical. Lye can cause severe chemical burns if it comes into contact with skin or eyes. According to the Soap Guild, it’s important to wear protective gear like goggles, gloves, long sleeves and pants when working with lye
The first step is preparing your workspace. Brambleberry recommends covering surfaces with plastic or newspapers in case of any spills. Also have a bowl of vinegar on hand, which can help neutralize lye on contact. Work in a well-ventilated area since lye can cause respiratory irritation. Never add water to lye as it can violently splatter. Always add lye to water slowly and stir carefully.
Per Soap Queen, if lye does spill on your skin, flush the area with water and vinegar for 20 minutes. For eye exposure, flush with water for 20 minutes. Seek medical attention for serious burns. Store lye in clearly labeled, airtight containers out of reach of children and pets. With proper precautions, lye can be handled safely for DIY soapmaking.
Cold Process
The cold process method is the most common way to make soap from scratch. It involves combining oils/fats with a lye solution at room temperature. Once combined, the mixture goes through saponification, where the lye reacts with the fatty acids in the oils to form soap and glycerin. Here is an overview of the basic cold process steps:
First, the lye solution is prepared by mixing sodium hydroxide (lye) with water and allowing it to fully dissolve. The oils/fats are melted if solid at room temperature. When both mixtures are ready, the lye solution is slowly poured into the oils, blending stick blend or whisk to emulsify. Adding the lye to the oils initiates saponification. The mixture will go through “trace” – becoming thick like a pancake batter.
Next, fragrances, colors, or additives can be mixed in. The soap is poured into molds to setup and harden. Cold process soapmaking requires soap to sit and cure for 4-6 weeks. This allows excess water to evaporate out and the soap molecules to rearrange and harden. The cured bars of cold process soap will be hard, mild, and ready for use.
The cold process method allows full customization and control over the oils, additives, and qualities of the finished soap. It requires safety precautions when using lye. With some patience, practice, and care, homemade cold process soap can be created in small batches from scratch. (Source)
Hot Process
Hot process is an alternative method of making soap that speeds up saponification through the application of heat. Instead of waiting 4-6 weeks for cold process soap to fully cure, hot process soap can be used almost immediately (source: https://www.soapguild.org/tools-and-resources/resource-center/71/hot-process-soap-beginners/).
The basic steps are:
- Melt oils and fats
- Mix lye and water to make a lye solution
- Combine oils and lye solution
- Cook the soap batter above 160°F
- Stir periodically until it reaches the vaseline stage
- Add fragrances, colors, or exfoliants
- Pour into molds
The additional heat speeds up saponification so it’s complete in 1-2 hours instead of weeks. This allows the soap to be used right away. The tradeoff is less time to add intricate color designs.
Additives
There are many different types of additives that can be used in soapmaking to customize the properties and qualities of the finished soap product. Some common additives include oils, colors, scents, botanicals, and exfoliants.
Additional oils like olive, coconut, or castor can be blended into the main soap oils to tailor the bar’s moisturizing qualities, bubbly lather, and hardness. Adding shea butter, cocoa butter, or mango butter will make the bar creamier. Source
Colorants and scents transform a plain bar of soap into something fun and inviting. Liquid soap dyes, micas, botanical powders, and fragrance oils infuse bars with vivid hues and lovely scents. Essential oils not only provide natural fragrance but also offer potential therapeutic benefits. Source
Botanicals like oatmeal, poppy seeds, dried flowers, and herbs can be mixed into the soap batter or decorated on top for visual and textural appeal. Exfoliants like sea salt, coffee grounds, and loofah can be swirled into bars to gently scrub and smooth the skin. Source
Conclusion
Soapmaking requires some basic chemistry knowledge to create a good bar of soap. The key ingredient that enables soap to clean is the fatty acids that are released when fats or oils are mixed with a strong alkali like lye. This process is called saponification. When the lye and fats react, the fatty acids are freed from the glycerin backbone of the triglycerides, creating soap. Choosing the right balance of oils and fats, and using the correct amount of lye, allows soapmakers to create bars with desired qualities. Understanding safety precautions when handling lye is also critical. While cold process and hot process methods differ slightly, the chemistry behind soapmaking remains the same. Additives can customize soap by adding color, scent, and skin benefits. In summary, grasping soap chemistry allows creators to make tailored, effective soaps.






