What Kind Of Reaction Is Burning Of Wax?
Burning wax is an example of a combustion reaction, which is defined as an exothermic chemical reaction between a fuel and an oxidant that produces oxides of carbon, water, heat, and light. Combustion reactions occur rapidly and give off heat and light in the form of a flame.
The burning of wax specifically refers to the exothermic chemical reaction between solid paraffin wax and oxygen gas. As the wax burns, it reacts with oxygen to produce carbon dioxide, water vapor, and energy in the form of heat and light. This makes burning wax an example of a combustion reaction.
Reactants
During the combustion reaction of burning a candle, the main reactant is the wax itself which provides the fuel source. The wax is typically made of long hydrocarbon chains such as paraffin wax. As the wax melts due to the heat of the flame, the hydrocarbon fuel mixes with oxygen from the air and vaporizes. Therefore, the reactants in burning a candle are the wax hydrocarbon fuel and oxygen gas from the surrounding air. As noted on the Weebly resource, “Reactants → Products. The chemical equation for the combustion (burning) of wax, expressed in words: wax + oxygen → carbon dioxide + water + energy (heat + light)” (source). So in summary, the two main reactants are the wax itself and oxygen from air.
Products
When a candle burns, the main products formed are carbon dioxide and water vapor. This can be represented by the chemical reaction:
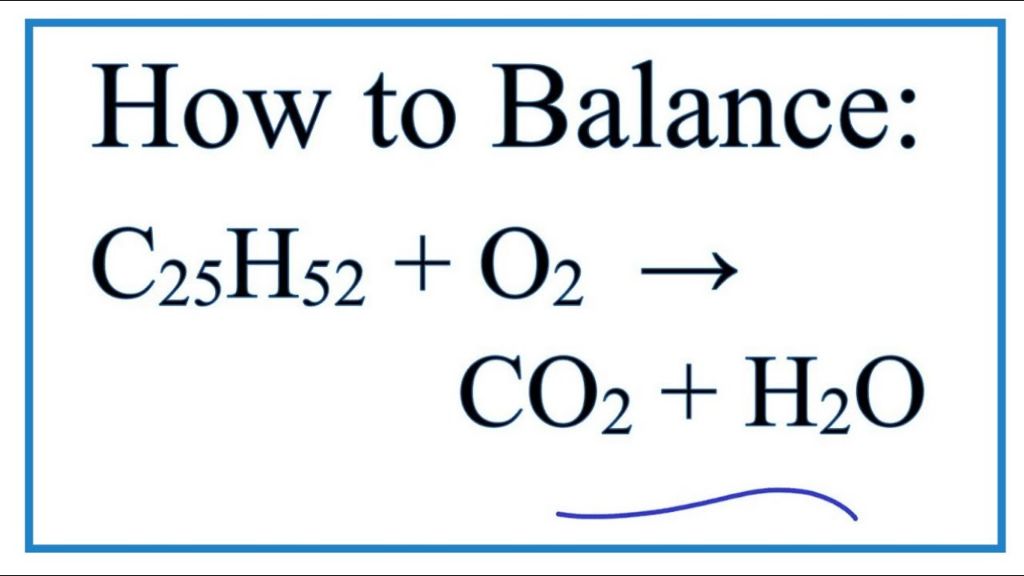
C25H52 + 38 O2 → 25 CO2 + 26 H2O
The hydrocarbon wax (C25H52) combines with oxygen (O2) from the air to produce carbon dioxide (CO2) and water vapor (H2O). As the candle burns, these gaseous products are released into the surrounding environment. The production of carbon dioxide and water vapor is evident from the flickering flame and pooling of liquid wax around the wick as the candle burns down.
In addition to gaseous products, a significant amount of heat energy is released during the combustion reaction. The high temperature of the flame indicates that burning wax is an exothermic process. The heat generated helps melt and vaporize more wax so that the reaction is self-sustaining. The release of energy as heat, light, and flame are characteristic of exothermic chemical reactions like combustion.
Overall, the main measurable products when a candle burns are carbon dioxide, water vapor, and heat energy. The production of these products indicates that a chemical change is taking place as the wax undergoes combustion.
Source: https://byjus.com/question-answer/8-name-the-products-formed-when-a-candle-burns-in-air/
Chemical Equation
The balanced chemical equation for the combustion (burning) of wax is:
C25H52 + 38 O2 → 25 CO2 + 26 H2O + energy (heat + light)
This chemical equation shows that 25 moles of wax (C25H52) react with 38 moles of oxygen gas (O2) to produce 25 moles of carbon dioxide (CO2), 26 moles of water (H2O) and energy in the forms of heat and light. The coefficients balance the number of atoms of each element on both sides of the equation.
Balancing chemical equations involves making sure there are equal numbers of atoms of each element on the reactant and product sides. This is important because atoms are conserved during a chemical reaction. The balanced equation allows us to visualize the quantitative relationships between reactants and products in a chemical reaction.
Source: https://www.breslyn.org/chemistry/balancing/public.php?eq_id=27
Exothermic Process
Burning wax is an exothermic process, which means that heat energy is released during the reaction. In exothermic reactions, the energy released is greater than the activation energy needed for the reaction to proceed. This results in an overall release of energy, usually in the form of heat (Endothermic vs. exothermic reactions).
Exothermic reactions differ from endothermic reactions, in which energy is absorbed from the surroundings. In endothermic reactions, the activation energy is greater than the energy released, resulting in an overall absorption of energy. Some examples of endothermic reactions are photosynthesis, melting of ice, and dissolving of baking soda in water. Exothermic reactions like burning wax release energy, while endothermic reactions absorb energy (Endothermic vs. exothermic reactions).
Activation Energy
The burning of wax requires an initial input of energy in order to get the reaction started. This is because wax molecules must first absorb enough energy to break their existing bonds before new bonds can form to produce carbon dioxide and water. This threshold amount of energy is known as the activation energy.
According to the University of North Florida, “The energy barrier is called the activation energy. The net energy of the reaction is the energy that is released as the methane burns. How do you overcome the activation energy barrier?” (Source). To initiate the combustion reaction, enough energy must be provided to overcome this activation energy barrier and destabilize the wax molecules.
Once the activation energy threshold has been met, the reaction becomes self-sustaining, as the energy released from breaking old bonds and forming new ones keeps the reaction progressing without additional external energy input. However, that initial activation energy is essential to starting the exothermic reaction of burning wax.
Reaction Mechanism
The burning of a candle wax follows a free radical chain mechanism. This involves initiation, propagation, and termination steps:
Initiation – The heat from the candle flame causes homolytic cleavage of a C-H or C-C bond in the hydrocarbon wax molecule to generate free radicals. For example:
C30H62 → C30H61• + H•
Propagation – The hydrocarbon free radical reacts with oxygen gas to form a peroxy radical. This peroxy radical can react with another hydrocarbon molecule to form a hydroperoxide and new hydrocarbon radical. These propagation steps continue the free radical chain.
C30H61• + O2 → C30H61OO•
C30H61OO• + C30H62 → C30H61OOH + C30H61•
Termination – Two free radicals combine to form nonradical products, ending the chain. For example:
C30H61• + HO2• → C30H61OH + O2
Overall, the alkyl free radicals react with oxygen to ultimately form carbon dioxide and water as the final combustion products (Source: https://www.thoughtco.com/where-does-candle-wax-go-607886).
Kinetics
The rate of the candle burning reaction depends on the kinetics, which refers to how fast or slow a chemical reaction takes place. The kinetics are determined by several factors:
Concentration of Reactants – The more wax present, the faster the reaction occurs as there are more molecules that can react. This is why candles burn quicker when they are new versus near the end.
Temperature – Increasing the temperature increases the reaction rate as the molecules have more kinetic energy and collide more often. The candle flame provides the heat needed to melt the wax and allow it to vaporize.
Catalysts – The wick acts as a catalyst, providing a surface for the liquid wax to vaporize from. This increases the rate of the reaction.
The overall rate law equation for the candle burning reaction can be written as:
Rate = k[Wax]^m[Oxygen]^n
Where k is the rate constant, and m and n are the orders of reaction with respect to wax and oxygen. Determining the kinetics provides insight into how variables like wick size and oxygen availability affect how quickly the candle burns.
Applications
Candles are one of the most common applications that utilize the burning of wax. The wax, often made of paraffin or beeswax, acts as the fuel source when lit by a wick (Source). As the wax melts and vaporizes, the vaporized molecules mix with oxygen and undergo combustion. This produces heat and light, allowing candles to provide illumination. Candles come in many sizes and shapes for decorative, religious, or practical lighting purposes.
Oil lamps also rely on the burning of fuel to produce light, historically using whale oil, olive oil, or kerosene. Like candles, the oil acts as the fuel source and the wick facilitates vaporization and burning. The controlled burning of the oil produces the lamp’s flame.
Other wax products like crayons and cosmetics also depend on the chemical properties of wax. Their physical structure and ability to melt at warm temperatures make waxes useful as a base material. The burning of wax has many applications throughout history and in modern daily life (Source).
Conclusion
In summary, burning of wax is an exothermic chemical reaction between a hydrocarbon wax and oxygen gas. The main reactants are paraffin wax molecules like C25H52 and O2 gas. The products are carbon dioxide, water vapor, and heat energy. The chemical reaction can be represented by the chemical equation:
C25H52 + 38 O2 → 25 CO2 + 26 H2O + heat
This reaction requires an input of activation energy to get started, after which the wax and oxygen rapidly react in a chain reaction mechanism. As the hydrocarbon burns, a significant amount of heat is released, making this highly exothermic. The reaction rate follows typical kinetics, speeding up with increasing temperature. This exothermic burning reaction has important applications for candles, but can also pose fire hazards. Overall, the burning of wax provides light and energy through a complex chemical process.



