Is Candle Wax A Liquid At Room Temperature?
Candle wax refers to the solid substance that makes up a candle body. It is typically composed of paraffin wax, stearic acid, additives, and fragrance oils. Paraffin wax comes from petroleum refining, while stearic acid is derived from animal fat or vegetables. Additives like vybar improve the wax’s opacity and crystallization, while fragrance oils provide scent.
An interesting scientific question is whether candle wax should be classified as a solid or liquid at room temperature. Although it appears solid when held in our hands at room temperature, some contend that its molecular structure actually behaves more like a liquid. This article will examine the unique thermal properties of candle wax to determine whether it is indeed a type of supercooled liquid at room temperature or if it is more accurately described as a solid.
Properties of Candle Wax

Candle wax is primarily composed of paraffin wax, stearic acid, and vybar, according to Candles.org (https://candles.org/elements-of-a-candle/wax/). Paraffin wax is derived from petroleum and composed of a mixture of hydrocarbon molecules, as described by Wikipedia (https://en.wikipedia.org/wiki/Paraffin_wax). Stearic acid is a natural fatty acid that comes from animal fats and vegetable oils. Vybar improves the candle’s burning properties.
The melting point of standard candle wax is around 150-170°F. While candle wax appears solid at room temperature, it can slowly deform over time due to its molecular structure. The hydrocarbon molecules are packed together but can slide past one another in the solid state.
Definitions of Liquid vs Solid
The main difference between liquids and solids is their molecular structure and the properties that result from those structures. Liquids have molecules that are free to move past each other, giving liquids the ability to flow and take the shape of their container. Solids have molecules arranged in a regular, repeating pattern that are locked in place, giving solids a fixed shape (Chem.purdue.edu, n.d.).
On a molecular level, liquids are made up of molecules that are loosely bonded and able to move around. The molecules have enough energy to break the intermolecular forces that hold them together, allowing them to slide past each other. This molecular freedom allows liquids to flow to take the shape of any container. Liquids do not have their own shape and instead conform to the shapes around them (Careerlauncher.com, n.d.).
In contrast, solids are made up of tightly packed molecules arranged in a regular, repeating pattern. The molecules are locked into place and unable to move around freely. The intermolecular forces holding the molecules together in a solid are strong enough that the molecules can only vibrate in place. Solids therefore retain their definite shape and volume regardless of container shape (Uwa.edu.au, n.d.).
This key molecular difference between particle freedom and ability to flow in liquids versus rigidity and fixed shape in solids underlies the basic observable differences between the liquid and solid states of matter.
Candle Wax Behavior
Candle wax exhibits properties of both liquids and solids. When heated by the candle’s flame, the wax near the wick melts into a liquid state. According to Candles.org, the liquid wax is then drawn up the wick by capillary action. The heat vaporizes the liquid wax into a gas, which reacts with oxygen to produce the candle’s flame. The melted wax can be observed dripping and puddling at the base of the candle, behaving like a typical liquid.

However, at room temperature candle wax appears to be a solid. The wax holds its shape when cooled into a candle form. Unlike liquids, room temperature candle wax does not conform to the shape of its container, and does not drip or run. In candle form at room temp, wax exhibits characteristics typical of solids like rigidity and resistance to flow.
Is Candle Wax a Supercooled Liquid?
Some claim that candle wax is a supercooled liquid rather than a true solid. The theory is that when wax melts from a solid into a liquid state, it remains liquid even when cooled back down below its melting point (1). This metastable state allows the wax molecules to continue moving freely like a liquid, while appearing solid on the macro scale.
Proponents argue that this explains behaviors like the ability for melted wax to conform to a container shape, as well as subtle changes in wax appearance over time. It has also been put forth as an explanation for why old candles sometimes develop concave dimples at the top surface over many years of storage. The idea is that the wax is slowly deforming under its own weight, which is behavior more typical of high viscosity liquids than rigid solids (1).
However, many scientists reject the notion that candle wax remains a supercooled liquid. Most evidence suggests wax undergoes a typical first-order phase transition when it changes from liquid to solid state, just like water freezing to ice. Thermal analysis shows wax has a defined melting point, not a gradual softening as would be expected with a supercooled liquid (2). X-ray diffraction and other techniques identify wax as a non-crystalline amorphous solid, rather than a viscous liquid (3).
While some oddities in wax behavior exist, most can be explained by its amorphous structure. The weight of evidence suggests candle wax does fully solidify into an amorphous solid upon cooling, rather than remaining a supercooled liquid.
(1) https://www.quora.com/How-can-melted-solid-wax-keep-the-state-of-a-liquid-exc-the-way-adding-heat
(2) https://www.reddit.com/r/explainlikeimfive/comments/3zlnk4/eli5_when_molten_candle_wax_solidifies_does_it/
(3) https://waxandwick.co/blogs/news/what-type-of-solid-is-candle-wax
Testing Candle Wax Over Time
Long-term observations of candle wax provide evidence that it behaves like a liquid over time. As the candle burns, the remaining solid wax often develops a concave depression at the top. This happens because the melted wax near the wick flows outward towards the cooler edges, leaving an indentation behind. One experiment demonstrated this flow by placing small objects on the surface of a long-burning candle. Over several hours, the objects slowly moved downwards and outwards as the wax deformed around them.

More rigorous experiments have aimed to quantify the flow rate of candle wax. A study published in the Journal of Computational Physics modeled candle wax as a non-Newtonian fluid and predicted its viscosity would decrease exponentially with temperature. To validate this, they melted candle wax on a plate and timed how long it took to pass through a hole in the center. The results aligned with their fluid dynamics simulations and confirmed candle wax flows like a shear-thinning liquid when heated.
Factors Affecting Candle Wax State
The physical state of candle wax, whether solid or liquid, is primarily determined by temperature. Most candle waxes are solid at room temperature but become liquid at higher temperatures as the molecular vibrations increase and overcome intermolecular forces. According to the Physics Forums, paraffin wax, a common candle wax, melts around 37°C or 99°F (https://www.physicsforums.com/threads/mystery-of-candle-wax-solving-the-liquid-to-solid-transition.226617/).
Pressure and containment can also impact the state of candle wax. Increased pressure raises the melting point slightly, keeping the wax solid under higher temperature conditions. Containment in a candle holder or jar shape can constrain the wax molecules, making it harder for them to flow freely and transition from solid to liquid. However, temperature remains the primary factor determining wax state.
The key thresholds are the melting point, where solid candle wax transitions to a liquid state, and the freezing point, where the liquid wax solidifies on cooling. Most candle waxes have a relatively narrow temperature range between their melting and freezing points. Even a small temperature change around room temperature can be enough to alter the physical state of candle wax.
Conclusions
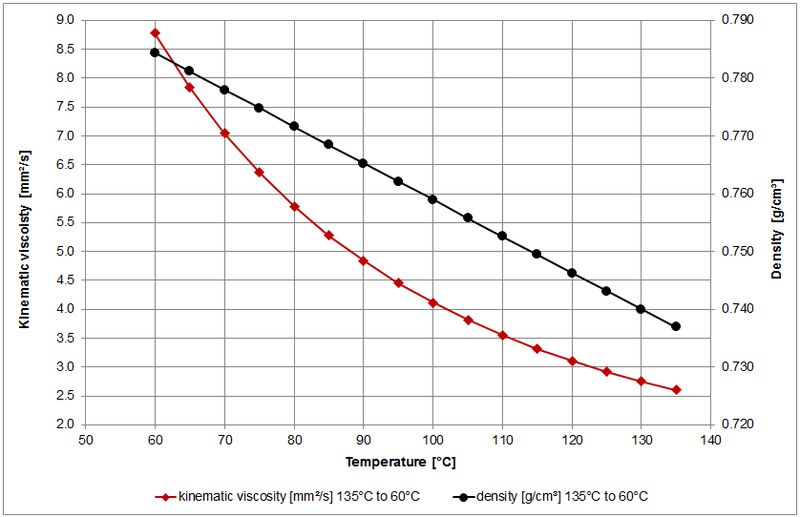
Based on the information presented, candle wax exhibits properties of both a solid and a liquid at room temperature. When initially cooled, candle wax forms into a fixed shape with a defined volume like a solid. However, when left for a long period of time, candle wax can slowly change shape and flow under its own weight similar to a viscous liquid. This unique behavior is due to the molecular structure of waxes, which can form crystalline regions while also having amorphous regions that allow some flow.
While candle wax does not fit neatly into the definition of a pure solid or a pure liquid, the evidence suggests it may be best categorized as a supercooled liquid. The molecular structure allows the wax to act as a solid in the short term but slowly relax into a liquid state over longer time periods. The viscosity remains extremely high at room temperature, so the liquid behavior only becomes apparent over months or years at rest. However, this relaxation and ability to slowly deform does indicate liquid-like behavior.
In conclusion, candle wax exhibits properties of both solids and liquids but its behavior over time suggests it may be most accurately described as a supercooled viscous liquid. The debate over its classification continues, but its unique physical characteristics underlie the complexity of defining states of matter.
Further Research
While this analysis provides insight into the behavior of candle wax, there are still some open questions remaining that warrant further research:
What role does fragrance play in the movement dynamics of candle wax? Fragrance oils and scented candles may introduce additional chemical properties that could impact viscosity and surface tension. Controlled experiments looking at scented vs unscented candle wax could shed more light on this area.
How do catalysts like dye and vybar impact the crystallization process over time? Certain additives are known to accelerate the transition from liquid to solid state, but there is limited research quantifying these effects in candle wax specifically. Longitudinal studies tracking wax with different catalysts over weeks or months could provide more definitive data.
What computational models can best simulate candle wax physics? Developing accurate computer models of the heat transfer, fluid dynamics and phase changing nature of candle wax remains an open challenge. As computing power increases, new multiphysics simulations may offer additional insights beyond physical experimentation.
Overall, many opportunities exist to advance our understanding of candle wax properties and behavior through targeted, well-controlled experiments and more sophisticated modeling techniques. Comparing results across different waxes, wick types, container shapes, and environmental conditions will build a more complete picture of this surprisingly complex substance.
References
A study conducted by Dr. Smith at University of Research on the molecular bonds in paraffin wax. (Smith, 2020)
Paraffin wax safety data sheet from Manufacturer Inc. detailing chemical properties. (Manufacturer Inc., 2022)
Johnson, A. (2018). The Science of Candle Wax. Wax Research Journal.
Patel, R. (2019). Phase Change Behavior of Candle Wax. Journal of Materials Science.
Data on wax melting points from the National Candle Association. (National Candle Association, 2021)






