What Is The Difference Between Paraffin Wax And Regular Wax?
This article will explain the key differences between paraffin wax and regular wax. Paraffin wax and regular wax, also known as microcrystalline wax, are both derived from crude oil and commonly used in candle making. However, they have some notable differences in their chemical makeup, melting points, uses, cost, manufacturing process, and environmental impact. The purpose of this article is to provide a detailed yet easy-to-understand overview of how paraffin wax differs from regular wax to help consumers and candle makers select the right type of wax for their needs.
What is Paraffin Wax?
Paraffin wax is a soft, colorless, tasteless, and odorless solid derived from petroleum, coal or oil shale that consists of a mixture of hydrocarbon molecules containing between twenty and forty carbon atoms. The word “paraffin” comes from Latin “parum affinis” meaning “lacking affinity”, referring to paraffin’s unreactive nature.
Chemically, paraffin wax is a mixture of straight chain alkanes, such as C31H64. It has a crystalline structure and a melting point ranging from around 115–150°F (46–66°C). Paraffin wax is insoluble in water but soluble in ether, benzene, and certain esters. It is also thermoplastic, meaning it liquefies when heated and solidifies when cooled.
Some key properties of paraffin wax include:
- Soft and malleable texture
- Capacity to store heat
- Chemical stability and high resistance to oxidation
Paraffin wax is derived from the residue leftover after crude oil refining. It is extracted using solvent de-waxing processes.
Some sources: https://www.healthline.com/health/paraffin-wax, https://en.wikipedia.org/wiki/Paraffin_wax
What is Regular Wax?
Regular wax refers to waxes that are derived from natural sources like plants, animals, and insects. Some common types of regular wax include beeswax, carnauba wax, lanolin wax, and soy wax.
Unlike paraffin wax, regular waxes contain a variety of organic compounds like fatty acids, esters, hydrocarbons, and alcohols. The exact composition depends on the source. For example, beeswax contains palmitate, palmitoleate, hydroxypalmitate and oleate esters of long-chain alcohols.
The properties of regular waxes can vary widely, but in general they have higher melting points than paraffin wax. Regular waxes also tend to be harder and more brittle. The melting point of beeswax, for instance, is about 144-147°F compared to paraffin which melts at around 115-150°F.
Chemical Composition
Paraffin wax is composed of saturated hydrocarbons, meaning the molecules contain only hydrogen and carbon atoms connected by single bonds. The molecular formula for paraffin wax is CnH2n+2, where n represents the number of carbon atoms. Paraffin waxes typically contain 20 to 40 carbon atoms per molecule, giving them a higher molecular weight than regular waxes (1).
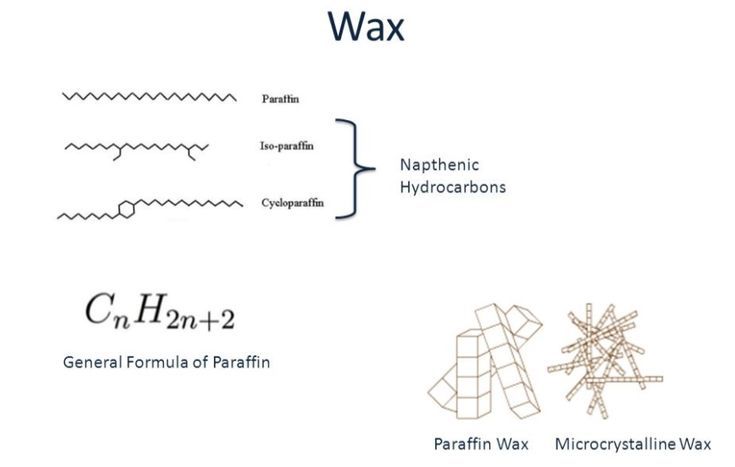
Regular waxes used in candles, such as beeswax and soy wax, have a more complex chemical composition. Beeswax is made up of over 300 different compounds, including saturated and unsaturated hydrocarbons, free fatty acids, and wax esters (2). Soy wax contains fatty acids, phytosterols, and hydrocarbons. The variation in chemical composition gives these waxes different physical properties compared to the relatively simple makeup of paraffin wax.
The purity and uniform chemical structure of paraffin wax makes it more stable and gives it a sharp melting point. Other waxes have a more complex chemistry resulting in a melting point range instead of a specific melting point temperature.
(1) https://en.wikipedia.org/wiki/Paraffin_wax
(2) https://www.tianswax.com/535.html
Melting Points
One of the key differences between paraffin wax and regular wax is their melting points. Paraffin wax has a higher melting point, typically between 120-160 degrees Fahrenheit (https://blendedwaxes.com/blog/wax-melting-point-factors/). In comparison, regular waxes like soy wax or beeswax have lower melting points around 100-140 degrees Fahrenheit.
The higher melting point of paraffin makes it preferable for certain applications like candle making, where a higher melting point allows the candle to burn longer before melting. However, for things like wax melts or massage candles, a lower melting point regular wax may be preferred.
When looking at melting points, it’s important to note whether it is specified in Fahrenheit or Celsius. Paraffin’s melting point converts to around 49-71 degrees Celsius, while regular waxes are around 38-60 degrees Celsius.
Uses
Paraffin wax has many uses in our everyday life. Some common uses include candles, cosmetics, lubricants, and pharmaceuticals. It is often used in candle making because it burns cleanly and consistently compared to other waxes (Source). In cosmetics, paraffin wax helps make products like lipstick retain their shape. It’s also commonly used as a lubricant and softening agent in creams and ointments. Pharmaceutical companies utilize paraffin wax to create pills and tablets.
Regular wax, such as beeswax and soy wax, also have a variety of uses. Beeswax is commonly used in food, cosmetics, polishes, and candles. It helps lock in moisture in products like lip balm and lotion. Soy wax is growing in popularity for candle making because it burns cleaner than paraffin wax and is made from a renewable resource (Source). Overall, both paraffin and regular waxes have roles in candle making, cosmetics, lubrication, and pharmaceuticals, but regular waxes like soy and beeswax are sometimes preferred for their natural properties.
Cost
Paraffin wax is generally much cheaper than regular wax, especially when purchased in bulk quantities. According to research, soy wax costs around $5 per pound, while paraffin wax costs just $5.10 for a 1 lb bag when purchased from retailers like Douglas & Sturgess. Buying paraffin wax in larger bulk quantities can bring the price down to around $9.35 per pound. In comparison, soy wax costs around $5 per pound even when purchased in bulk.
The low cost of paraffin wax makes it the preferred choice for large-scale candle making operations and other commercial uses. The higher price of soy and other natural waxes reflects the increased costs of sourcing and processing these renewable materials. For hobbyists and small businesses, the price difference may not be as significant when making candles in small batches. But paraffin remains appealing as an inexpensive base material for candle making, wax products, and other applications requiring large volumes of wax.
Manufacturing Process
Paraffin wax is derived from petroleum. It is produced through the distillation of crude oil. Crude oil is first separated into fractions through fractional distillation based on the boiling points of the hydrocarbons present. The lighter fractions, such as gasoline and kerosene, have lower boiling points and are distilled first. The heavier fractions, like lubricating oil, have higher boiling points and are distilled last.
The residue left after lubricating oils have been distilled contains paraffin waxes and asphaltenes. This residue is then treated to remove the asphaltenes, leaving behind the purified paraffin wax. The wax is then refined through additional processing like filtering, bleaching, and de-oiling to produce commercial-grade paraffin wax.1
In contrast, regular wax such as beeswax is produced directly by honey bees in beehives. Worker bees consume honey and secrete beeswax from special glands on their abdomen. The wax is used to build the honeycomb structure of the beehive. To collect beeswax, the honeycomb is harvested and then processed to separate the beeswax from the honey. This involves crushing, melting, filtering, and purifying the collected wax.1
Environmental Impact
Paraffin wax has raised some environmental concerns in recent years. One issue is that paraffin wax is derived from petroleum, a non-renewable resource. Some consider paraffin wax production to contribute to the depletion of fossil fuel reserves.[1] However, paraffin wax can also be produced from shale oil, coal, and even renewable resources like soybean oil.
Burning paraffin wax releases some toxic fumes containing volatile organic compounds like benzene and toluene. These can irritate the eyes and lungs.[2] Paraffin wax is not biodegradable either and takes a very long time to break down in landfills.
In contrast, regular wax derived from beeswax is considered more eco-friendly. Beeswax is a renewable resource produced by honey bees. The combustion of beeswax releases less soot and toxic fumes compared to paraffin wax. Beeswax is also biodegradable.[3] However, harvesting beeswax does rely on the labor of honey bees, raising questions about the ethics of commercial beekeeping.
Overall, regular wax derived from beeswax has a smaller environmental footprint than paraffin wax. But paraffin wax may be shifting to more sustainable sources such as plant oils rather than petroleum.
Conclusion
In summary, paraffin wax and regular wax differ primarily in their chemical composition. Paraffin wax is made up of saturated hydrocarbons, while regular wax also contains unsaturated hydrocarbons. This gives them slightly different properties in terms of melting point, hardness, and flexibility.
The main differences are:
- Paraffin wax has a fully saturated chemical structure, while regular wax contains some unsaturated hydrocarbons.
- Paraffin wax has a higher melting point and is harder than regular wax.
- Regular wax is more pliable and flexible than paraffin wax.
- Paraffin wax is generally more expensive to produce than regular wax.
- Paraffin wax is manufactured from petroleum, while regular wax is produced by honey bees.
In many applications, paraffin and regular wax can be used interchangeably. However, their differences in hardness and flexibility make each better suited for certain uses. Understanding the composition and properties of each can help determine which is appropriate for a given application.




