What Happens After Wax Melts?
Candles are a popular household item used for lighting, fragrance, and decoration. When a candle is lit, the wax melts from a solid to a liquid state due to heat from the flame. The melting process seems simple, but there are actually several interesting phenomena occurring. This article will provide an overview of what happens when candle wax melts, looking at the melting point, wick ignition, vaporization, burning, and more. We’ll explore the key concepts around this everyday process that allows candles to provide their comforting glow.
Composition of Wax
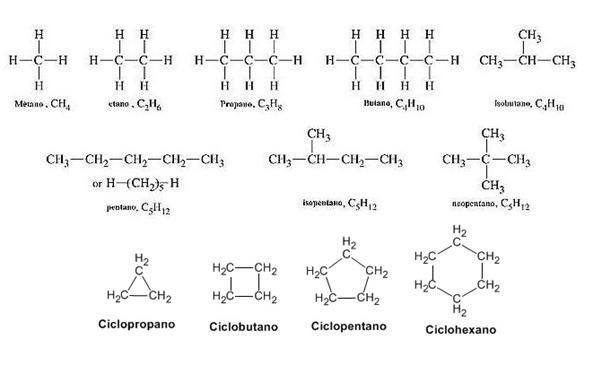
Wax is made up of long hydrocarbon chains known as alkanes or paraffins. These hydrocarbon chains typically contain between 20 and 40 carbon atoms. The most common alkane in wax is octacosane, which contains 28 carbon atoms chained together. Other alkanes present include hexacosane (C26), heptacosane (C27), nonacosane (C29), triacontane (C30) and dotriacontane (C32).
In addition to alkanes, wax may contain other organic compounds like fatty acids, fatty alcohols, and esters. The exact composition depends on the source of the wax. Beeswax contains esters and fatty acids, while paraffin wax is composed almost entirely of alkanes.
The long hydrocarbon chains in wax pack together well, giving wax its characteristic hardness and high melting point. The strength of the intermolecular van der Waals forces between the chains accounts for wax’s physical properties.
Melting Point
The melting point of a wax refers to the temperature at which it transitions from a solid to a liquid state. This property is determined by the composition and structure of the hydrocarbon chains that make up the wax.
Waxes are composed of long hydrocarbon chains, which are made up of carbon and hydrogen atoms. The length of these chains and whether they are branched or straight chained affects the melting point.
Longer straight chains result in higher melting points, while shorter and branched chains have lower melting points. This is because longer chains can pack together more tightly in solid form, requiring more energy (higher temperatures) to overcome the attractive forces between molecules and melt.
Paraffin wax, made up of straight chained hydrocarbons in the C20 to C40 range, has a melting point of 46°C to 68°C. In comparison, beeswax, which contains branched chains and shorter hydrocarbons, melts at lower temperatures of 62°C to 64°C.
The melting point is an important property of waxes that determines their applications. Candles require waxes with melting points suitable for stable structure when solid but easy melting when burning. Knowledge of wax composition and structure allows prediction and customization of the melting point.
Phase Change
Wax undergoes a phase change from solid to liquid during the melting process. This occurs because heating wax provides energy to overcome the intermolecular forces that hold the wax molecules together in a rigid, solid structure. The main intermolecular forces in wax are van der Waals forces, which are relatively weak interactions between molecules.
As heat is applied to solid wax, the molecules gain kinetic energy and begin vibrating more intensely. When enough energy is provided to overcome the van der Waals forces, the molecules can slide past each other and the rigid structure of the solid breaks down. This allows the molecules to move more freely and take on liquid properties. The temperature at which this occurs is known as the melting point, which varies for different types of wax. Once the phase change to liquid is complete, the wax can then be absorbed into the wick.
Absorption into Wick
Once the wax melts from the heat of the flame, it is then absorbed into the wick through a process called capillary action. Capillary action refers to the ability of a liquid to flow into narrow spaces without the assistance of external forces like gravity. This phenomenon occurs because of intermolecular forces between the liquid and the surrounding surfaces.
In a candle, the wick is made up of many tiny fibers bundled together, creating lots of empty spaces between the fibers. When the melted wax comes in contact with the wick, it gets pulled into these spaces. This is because of adhesion and surface tension – the wax adheres to the surface of the wick fibers and is pulled inward. The narrower the spaces in the wick, the farther the wax can travel upward through capillary action.
The wax continues to be absorbed into the wick until it reaches the glowing tip where the flame is. This wax absorbed into the wick acts as fuel for the flame to keep burning. The capillary action ensures a continuous supply of melted wax is transported up the wick to sustain the flame as long as there is wax to melt in the pool.
Wick Ignition
Once the wax melts and is absorbed into the cotton fibers of the wick, the wick reaches a temperature where it can ignite and sustain a flame. This occurs because the wax-saturated wick acts as fuel for the flame. As the melted wax travels up the wick through capillary action, it vaporizes at the top of the exposed wick. The vaporized wax molecules mix with oxygen in the air, creating a combustible fuel-air mixture around the wick. When the wick reaches the ignition temperature of the wax fuel (around 400°F or 200°C), the heat energy is enough to initiate combustion, and the wax vapors ignite.
The flame is then sustained by the continuous supply of melted wax fuel moving up the wick. As the wax vaporizes at the top of the wick, it feeds the flame. Meanwhile, the melted wax pool is replenished by more solid wax melting. This constant fuel supply keeps the wick hot enough to maintain the combustion reaction. The flame provides the heat energy needed to keep vaporizing and igniting the wax. This consistent evaporation and burning of wax sustains the flame until the fuel is depleted when all the solid wax has melted and burned up.
Melted Wax Pool
As the heat from the flame melts the solid wax at the top of the candle, a pool of liquid wax forms around the wick. This melted wax pool is key to sustaining combustion. The pool acts as a reservoir, allowing the wick to continuously absorb liquid fuel. As solid wax at the edge of the pool melts, the liquid wax flows inward toward the wick.
The melted wax pool can vary in size and shape depending on factors like the candle’s shape, wax composition, and burn rate. Wider containers allow for larger melt pools. The wax pool typically forms a concave shape, deeper in the center near the wick. This is because the heat is most intense directly above the flame. The melted wax flows outward from the hot spot in a radial pattern.
As the candle continues to burn, the melt pool expands outward. This exposes more solid wax to melting, providing an ongoing fuel supply. Most candles are designed so the melt pool stops spreading once it reaches the edges of the container. This prevents wax from overflowing.
Wax Vaporization
As a candle burns, the melted wax at the top of the candle wick gets hotter and eventually reaches the boiling point of the wax. When the wax reaches its boiling point, the heat causes individual wax molecules to break free of the liquid wax and evaporate into a gaseous vapor state.
The vaporization of wax molecules is key to sustaining the candle’s flame. As wax vaporizes from the liquid wax pool into a gas, these vaporized wax molecules provide additional fuel for the candle flame through combustion. The heat of the flame keeps the wax at the wick continuously vaporizing to supply this fuel.
Interestingly, different types of wax have different boiling points based on their molecular composition. For example, paraffin wax has a lower boiling point than beeswax, so paraffin vaporizes more readily. The wax boiling point impacts the rate of vaporization and burn time. But in all cases, the conversion of liquid wax into gaseous vapors through vaporization provides the necessary fuel source for the candle flame. The vaporized wax burns and releases heat, continuing the cycle of melting, vaporizing, and burning the wax fuel.
Wax Burning
As the melted wax pool reaches the flame, the heat causes the liquid wax molecules to transition from liquid to gas, through a process called vaporization. The wax vapors rise through the air and mix with oxygen. When these hot wax vapors reach the flame, combustion occurs.
Combustion is a high-temperature exothermic chemical reaction between a fuel and an oxidant, usually atmospheric oxygen. In the case of a burning candle, the hot wax vapors act as the fuel, while the oxygen in the air acts as the oxidant.
Specifically, the hydrogen and carbon atoms within the wax molecules rapidly react with the oxygen atoms to form water vapor (H2O) and carbon dioxide (CO2). This chemical reaction gives off heat and light, which we observe as the flame. The energy released provides the activation energy to keep the chain reaction going as long as there is fuel available.
The visible yellow and orange colors of the candle flame come from tiny particles of unburnt carbon called soot. The blue part of the flame is hotter and complete combustion is occurring there. The hot gases produced rise upward carrying the soot with them, leaving less soot at the hottest part of the flame.
Conclusion
Overall, a lot happens after wax melts. The process starts with wax transitioning from a solid to a liquid through melting caused by heat from the flame. The melted wax gets absorbed into the wick through capillary action. The wax-saturated wick combusts, which provides the heat to continue melting more wax. This forms a melted wax pool that fills the candle container. Some liquid wax also vaporizes at the high temperature and combusts. The full sequence is: solid wax melts into a liquid, liquid wax gets absorbed by the wick, the wick ignites, melted wax pools while some vaporizes, and the cyclical burning continues consuming wax and releasing energy. Through this detailed process of phase changes and chemical reactions, melted wax provides the fuel for a controlled flame in candle wax burning.