Is Wax Soluble In Isopropyl Alcohol?
The topic examines whether wax is soluble in isopropyl alcohol. To understand solubility, we first need background information on the chemical properties of the two substances in question – wax and isopropyl alcohol.
Wax refers to a class of organic compounds composed of long-chain fatty acids that are solid at room temperature. Examples of waxes include paraffin wax made from petroleum, beeswax from bees, and synthetic wax made from polyethylene. [https://www.ncbi.nlm.nih.gov/books/NBK208299/] The waxes differ in their molecular structure and hydrocarbon chain length, which can influence solubility.
Isopropyl alcohol (also called isopropanol or 2-propanol) is a colorless liquid that dissolves in water. It has a molecular formula of C3H8O and is made by combining water and propene. [https://pubs.acs.org/doi/10.1021/i560152a019] Isopropyl alcohol is widely used as a solvent and has the ability to dissolve many organic compounds, especially oils.
Definition of Solubility
In chemistry, solubility refers to the ability for a given solute to dissolve in a solvent. It is defined as the maximum amount of solute that can dissolve in a specific amount of solvent at a given temperature and pressure to produce a saturated solution (1).
Solubility is often measured as the grams of solute per milliliter of solvent (g/mL). For example, if 1 g of a solute can dissolve in 100 mL of solvent at 20°C, the solubility of that solute is 0.01 g/mL. The higher the solubility, the more solute can dissolve in the solvent before reaching saturation (2).
Solubility depends on various intermolecular interactions between the solute and solvent, such as polarity, hydrogen bonding, van der Waals forces, and more. These interactions determine how compatible the solute is with the given solvent.
Chemical Properties of Wax
Wax is comprised of esters, hydrocarbons, free fatty acids, and alcohols. It has a unique chemical composition that differs based on its origin. Beeswax consists primarily of esters of fatty acids and alcohols, along with some free fatty acids and alcohols. Paraffin wax is comprised of straight chain hydrocarbons usually ranging from C20 to C40. Microcrystalline waxes contain branched and cyclic hydrocarbons as well (https://onlinelibrary.wiley.com/doi/abs/10.1002/1438-9312%28200207%29104%3A7%3C387%3A%3AAID-EJLT387%3E3.0.CO%3B2-C).
The chemical structure of wax gives it unique physical properties. It is insoluble in water due to its non-polar hydrocarbon composition. Wax is also thermoplastic, meaning it melts when heated and solidifies when cooled. This reversible melting allows wax to transition between a liquid and solid state.
Chemical Properties of Isopropyl Alcohol
Isopropyl alcohol, also known as isopropanol or 2-propanol, has the chemical formula C3H8O. Its IUPAC name is propan-2-ol. The structure of isopropyl alcohol consists of a 3-carbon backbone with an OH group attached to the central carbon atom. This makes it a secondary alcohol.
Some key chemical properties of isopropyl alcohol are:
- It is flammable, with a flash point of 11.7°C (https://testbook.com/chemistry/isopropyl-alcohol). This makes it highly flammable.
- It can dissolve in water due to the polarity provided by the OH group. The solubility in water is appreciable, around 50 g per 100 mL of water (https://byjus.com/chemistry/isopropyl-alcohol/).
- It readily oxidizes to form acetone in the presence of oxidizing agents like chromic acid or nitric acid (https://testbook.com/chemistry/isopropyl-alcohol). The oxidation involves the conversion of the secondary alcohol to a ketone.
- It reacts with sodium to form hydrogen gas (https://baochemicals.com/what-is-isopropyl-alcohol-ipa-uses-and-chemical-properties/). This reaction is characteristic of alcohols.
The chemical properties of isopropyl alcohol allow its use as a solvent and disinfectant. The polarity provided by its hydroxyl group gives this alcohol amphiphilic properties.
Polarity
Polarity refers to how evenly distributed the electric charge is across a molecule. Substances with symmetrical molecular structures, like carbon dioxide, have their electric charges evenly distributed and are considered nonpolar. Asymmetrical molecules, like water, have an uneven charge distribution and are polar. The uneven distribution of charge makes one part of a polar molecule partially positive and another part partially negative.
Polarity affects solubility because “like dissolves like.” Polar substances tend to be soluble in other polar substances. This is because the partially positive and partially negative ends of polar molecules are attracted to each other via hydrogen bonds. Nonpolar substances like oils and waxes have a hard time interacting with polar solvents like water. Their molecules lack charged regions that can form attractions. However, nonpolar substances are soluble in other nonpolar substances because their molecules can freely interact and mix together through London dispersion forces.
In summary, similar polarities lead to solubility while vastly different polarities cause insolubility. The polarity of both the solute and solvent impacts whether they will mix together in solution.
Source: http://gzscienceclassonline.weebly.com/5-polarity-and-solubility.html
The Solubility of Wax in Isopropyl Alcohol
The solubility of wax in isopropyl alcohol depends on the type of wax. Most waxes like paraffin wax, beeswax, and soy wax are insoluble in isopropyl alcohol, which means they will not dissolve. This is because waxes are non-polar hydrocarbons while isopropyl alcohol is a polar solvent (Isopropyl alcohol and beeswax).
However, some waxes like carnauba wax and polyethylene wax have a small degree of solubility in isopropyl alcohol, meaning they are slightly soluble. This is due to their unique chemical properties that allow minor interactions with the polar isopropyl alcohol molecules (Is Wax Soluble in Isopropyl Alcohol (IPA)?).
Overall, most common waxes are insoluble while some specialized waxes have limited solubility. So in general, wax and isopropyl alcohol are not soluble and wax will not dissolve in isopropyl alcohol. The main reason is the polarity difference between the molecules preventing proper interactions.
Factors That Impact Solubility
There are several key factors that impact the solubility of a solute like wax in a solvent like isopropyl alcohol, including temperature, pressure, concentration, and chemical properties of the substances involved.
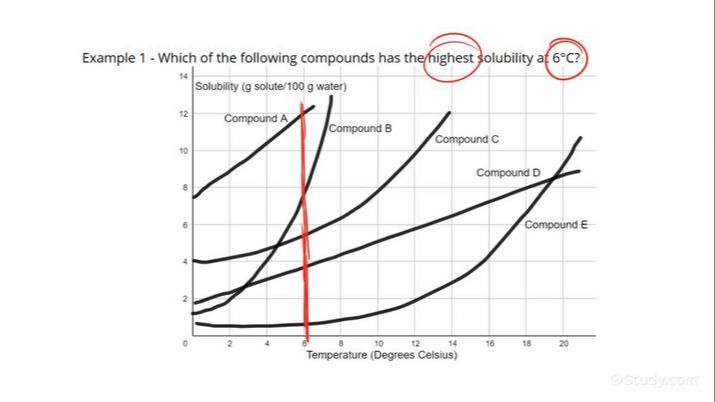
Temperature is one of the most important factors. Typically, solubility increases as temperature increases. This is because at higher temperatures, both solute and solvent particles move faster, collide more frequently, and overcome intermolecular forces more easily, allowing more solute to dissolve. For example, more sugar dissolves in hot water compared to cold water.
Increased pressure can also increase solubility, especially for gaseous solutes in liquid solvents, according to Le Chatelier’s principle. The pressure forces more gas molecules to dissolve in the liquid phase.
Concentration affects solubility as well. A saturated solution is one in which no more solute can dissolve at a given temperature. Adding more solute beyond saturation will result in undissolved solid remaining. Diluting a solution or removing solute decreases concentration, allowing more solvent particles to interact with solute, increasing solubility.
Finally, the chemical properties of the solute and solvent impact solubility. Generally, polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. Solubility improves when solvent and solute exhibit similar intermolecular forces that allow favorable interactions.
For more details, refer to this overview on factors affecting solubility: Solubility and Factors Affecting Solubility
Other Solvents for Wax
Wax can dissolve in several other solvents besides isopropyl alcohol. Some commonly used solvents for wax include:

- Mineral spirits – Mineral spirits are a petroleum-based solvent that can effectively dissolve wax. They have strong solvency power and can break down the crystalline structure of wax. (Source)
- Turpentine – Turpentine is another petroleum-derived solvent that can dissolve wax. It has been widely used as a solvent for wax in applications like wood finishing. (Source)
- Naphtha – Naphtha is a broad term referring to several volatile hydrocarbon liquids. Certain types of naphtha make effective solvents for dissolving wax. (Source)
When selecting an appropriate solvent, it is important to consider factors like compatability, flammability, toxicity, and environmental impact. The solvent must be able to fully dissolve the wax for the intended application.
Applications
The solubility of wax in isopropyl alcohol has several useful applications:
- In cosmetics, isopropyl alcohol is often used to dissolve wax-based ingredients like beeswax or paraffin. This allows waxes to be incorporated into products like lotions, creams, and lip balms (https://en.wikipedia.org/wiki/Wax).
- Wax coatings are sometimes applied to fruits and vegetables to help retain moisture. The wax can be removed by washing the produce in isopropyl alcohol solutions (https://pubmed.ncbi.nlm.nih.gov/17123917/).
- Isopropyl alcohol can dissolve wax buildup inside industrial equipment like boilers and heat exchangers. This helps remove accumulated deposits and improve efficiency (https://www.adc-solution.com/en/about-waxes/).
- Artists can use isopropyl alcohol to clean brushes and dissolve dried wax leftover from oil painting or encaustic techniques.

Conclusion
In conclusion, wax is soluble in isopropyl alcohol due to the polarity differences between the two compounds. Wax is nonpolar while isopropyl alcohol is polar, allowing the polar alcohol molecules to interact with and dissolve the nonpolar wax molecules. Factors like temperature and wax composition impact solubility, with higher temperatures and lighter hydrocarbons improving solubility. While isopropyl alcohol is a common solvent for wax, other solvents like turpentine and limonene can also dissolve wax effectively. Understanding wax solubility allows important industrial and commercial applications like removing wax from equipment, dissolving lipstick stains, and thinning waxed wood finishes.
Key points covered include:
- Wax is nonpolar while isopropyl alcohol is polar, enabling solubility.
- Temperature, wax composition, concentration affect solubility.
- Isopropyl alcohol dissolves wax through polarity differences.
- Other solvents like turpentine also dissolve wax.
- Wax solubility enables removing wax, thinning finishes, cleaning.
By examining the chemical properties and polarity differences between wax and isopropyl alcohol, this content shows why wax can dissolve in the alcohol solvent and highlights the main factors that impact solubility. Understanding the solubility interactions provides helpful insight for key industrial and commercial applications. The content leaves readers with a clear summary of the concepts discussed.




