Is Detergent Soap A Base?
Detergent soap is a type of cleaning agent made from synthetic detergents rather than natural soap. Detergent soaps are commonly formulated for use in laundry, dishwashing, and household cleaning. They work by reducing the surface tension of water, allowing it to penetrate fabric and surfaces more effectively to loosen dirt and oils. Unlike traditional soap made from animal fat or vegetable oils, detergent soaps do not form an insoluble precipitate in hard water and can produce more suds. They also often contain added enzymes and whitening agents to boost cleaning performance. While the terms “soap” and “detergent” are sometimes used interchangeably, detergent soap has different chemical properties from traditional soap despite serving a similar cleaning function.
Chemical Composition
Detergent soaps typically contain several main ingredients that provide cleaning power. These include:
- Surfactants – These reduce surface tension and allow water to mix with oils and grease. Common surfactants in detergents include sodium lauryl sulfate and linear alkylbenzene sulfonates. Surfactants make up 10-30% of detergents (https://www.thespruce.com/how-laundry-detergent-ingredients-work-2146619).
- Builders – Builders help remove calcium and magnesium ions which can make water harder. Builders like phosphates (now banned in many places) and zeolites help surfactants work better. Builders can be 20-50% of the detergent.
- Bleaches – Bleaches like hydrogen peroxide whiten fabrics by oxidizing stains. Chlorine bleaches are effective but harsh.
- Enzymes – Enzymes like protease break down protein-based stains. Amylase enzymes target starches.
- Fragrances – Fragrances give detergents a pleasant smell but don’t contribute to cleaning power.
- Dyes and optical brighteners – These ingredients don’t clean but make clothes look cleaner.
The combination of these ingredients gives detergent soaps their cleaning power. The main cleaning agents are surfactants and builders.
pH of Detergent Soaps
The pH of most detergent soaps tends to be slightly alkaline, in the range of 8-10 on the pH scale. According to research, the pH of laundry detergent pods is mainly between 7.0-8.0 (neutral) and 5.0-7.0 (mildly acidic) (Chan, 2019). Although some detergents may have a pH around 5-6, most are formulated with a pH between 7-10 because this higher alkalinity helps remove grease and oil-based stains more effectively (ACleanerWorld, 2019). Highly acidic or alkaline cleaners could potentially damage fabrics over time, so most mainstream detergents aim for a mildly alkaline pH that balances cleaning power with gentleness.
Properties of Bases
A base is defined as a substance that produces hydroxide (OH-) ions when dissolved in water. Bases have some key characteristic properties:
- Bases have a pH greater than 7, indicating they are alkaline.
- Aqueous solutions of bases feel slippery or soapy.
- Bases can neutralize acids and raise the pH of acidic solutions.
- Strong bases fully dissociate into ions in aqueous solutions.
- Weak bases only partially dissociate in water.
- Bases react with acids to form salts and water.
- Common bases include metal hydroxides like sodium hydroxide and potassium hydroxide.
In summary, bases are defined by their ability to produce OH- ions and have a pH above 7. They feel slippery, react with acids, and can neutralize acidic solutions (1).
Detergent Soap as a Base
Detergent soaps are considered bases because they have a pH above 7. Most detergent soaps have a pH between 8-10, making them alkaline. The alkalinity comes from the chemical ingredients used to make detergent soaps.
Detergent soaps contain surfactants, which are the main cleaning agents. Many common surfactants are derivatives of strong bases like sodium hydroxide or potassium hydroxide (lye) [1]. When these bases react with fatty acids from oils during saponification, they produce the salt-based surfactants found in detergents.
For example, sodium hydroxide reacts with fatty acids to produce sodium salts of the fatty acids. These sodium salts are amphoteric surfactants that act as bases when dissolved in water. The sodium salts raise the pH, making detergent solutions alkaline [2].
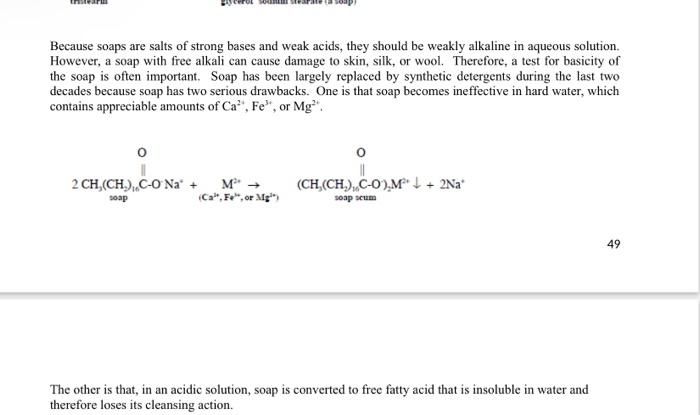
In addition to surfactants, detergents contain alkaline builders like sodium carbonate (washing soda) that boost cleaning power. Builders help remove calcium and magnesium ions that interfere with surfactant activity. This further contributes to the base properties of detergents [3].
The combination of surfactant salts and alkaline builders is why commercial detergent soaps test as bases rather than neutral when dissolved in water. The base properties allow detergents to efficiently break down oils and grease while remaining gentle on fabrics.
[1] https://www.quora.com/Is-detergent-an-acid-or-a-base
[2] https://www.activatedeco.com/blogs/news/is-detergent-an-acid-or-base
[3] https://www.themacbath.com/blog/2016/6/27/back-to-basics-what-is-soap
Saponification Process
Saponification is the chemical process that produces soap. It involves converting fats or oils into soap by mixing them with an alkaline solution such as lye. The fats and oils contain fatty acids that react with the lye, causing a chemical reaction called saponification.
Specifically, the process involves three main steps:
1. Saponifiable fats or oils are mixed with lye (sodium hydroxide NaOH) in a soap making pot. Common fats used include oils like olive, coconut or palm oils. The lye acts as a catalyst.
2. As the mixture is heated, the hydroxide ions of the lye interact with the fatty acids in the fats or oils. This breaks down the oils through hydrolysis into glycerol and crude soap. The crude soap consists of sodium salts of the fatty acids.
3. These sodium salts, or soaps, are then separated from the glycerol by adding salt to the mixture. The soaps will float to the top and can be skimmed off, while the glycerol sinks to the bottom. This is called salting out the soap.
So in summary, saponification is the base-driven hydrolysis of esters, turning fats/oils into soap through a chemical reaction with lye. The end products are glycerol and crude soap. This reaction produces the cleansing agent that gives soaps their ability to emulsify oils and hold water.[1]
[1] https://potagersoap.com/blogs/news/what-is-saponification
Surfactant Properties
Detergents like laundry detergent work by lowering the surface tension of water, which allows the detergent to penetrate fabrics more easily and lift dirt, oils, and grime from the fabric. According to the ScienceDirect overview on soap surfactants, the key property that allows detergents to effectively clean is their ability to lower surface tension (ScienceDirect). Detergents contain surfactant compounds that have both hydrophilic (water-loving) and hydrophobic (water-fearing) regions within the molecule. The hydrophilic regions interact with water while the hydrophobic regions interact with oils and grease. This dual-action allows the surfactants to act as a bridge between the oil/grease and the water, which loosens the dirt and pulls it away from the fabric.
The hydrophilic regions of the surfactant molecules are attracted to water molecules and form hydrogen bonds with them. This interaction makes it difficult for the water molecules to hydrogen bond with each other, which reduces the surface tension of the water dramatically. The lower surface tension allows the water to penetrate fabrics more thoroughly and lift away particulates. The hydrophobic regions of the surfactant molecules interact with oils and grease, surrounding dirt particles and prying them off surfaces. The dirt is held suspended in the water solution by the surfactant molecules. This overall reduction in water surface tension and bonding with oils is the key mechanism that gives detergents their cleaning power.
Cleaning Power
Detergent soaps are effective cleaning agents because they are alkaline, meaning they have a high pH. Most dirt, grime, oils, and greases that need to be cleaned are acidic in nature. According to the article “The Chemistry of Cleaning” on CM Monline, “An alkaline cleaner can emulsify acidic soils by reacting with the fatty acids in the soil to create a salt or soap that can be rinsed away.”
The article “Acids & Bases” on About Cleaning Products explains further: “Acidic cleaners attack and dissolve these types of stains, breaking them down and making them easier to remove. Bases do the opposite – they are good at removing stains that are acidic.”
Therefore, the alkaline properties of detergent soaps allow them to neutralize and dissolve acidic compounds like dirt, making detergent an effective cleaning agent. The high pH of detergent soaps breaks down grime and lifts it away from surfaces.
Environmental Effects
Detergent soaps can have negative impacts on the environment if not used properly. One of the biggest concerns is water pollution. Many detergents contain phosphates, which can promote excessive algal bloom when released into water sources. This algal bloom blocks sunlight and reduces oxygen levels, harming aquatic life (1).
Another environmental concern is that nitrogen-containing compounds in some detergents can lead to eutrophication, which is an increase in nutrients that reduce water quality. Detergents may also contain compounds that are toxic to aquatic organisms (2).
Some steps can be taken to reduce the environmental impact of detergents. Using phosphate-free and biodegradable detergents, only washing full loads, and not pouring unused detergent down the drain can help. Also, wastewater treatment facilities are now better equipped to remove phosphates before water is discharged back into the environment (3).
Overall, while detergent soaps can have negative environmental effects if used improperly, there are detergent options and washing practices that can greatly reduce any potential harm.
(1) https://www.kindlaundry.com/blogs/educational/impact-of-detergents-on-the-environment
(2) https://www.greenmatters.com/p/detergent-environmental-effects
(3) https://stppgroup.com/the-environmental-impact-of-laundry-detergent-how-to-make-a-greener-choice/
Conclusion
In conclusion, the evidence shows that detergent soaps can be classified as bases:
– Detergent soaps are made via the saponification process, which involves reacting fats/oils with a strong base like sodium hydroxide or potassium hydroxide. This produces surfactant molecules like sodium stearate that are the main cleaning agents in detergent soaps.
– These surfactant molecules contain an ionic end that dissolves in water, giving detergent soaps the ability to lower surface tension and emulsify oils and grease. This is characteristic of bases.
– Detergent soaps generally have a pH in the 9-10 range, making them alkaline. Bases by definition have pH values greater than 7.
– When dissolved in water, detergent soaps increase the concentration of hydroxide ions (OH-), which is another defining property of bases.
– Detergent soaps exhibit chemical properties similar to other common bases like sodium hydroxide and ammonia. They react with acids to form salts and water, and turn red litmus paper blue.
In summary, the chemical composition, pH, ionic properties, and reactivity all confirm that detergent soaps can be classified as bases. Their cleaning power comes from their alkaline, surfactant nature.






