What Is The Boiling Point Of Candle Wax?
Candle wax refers to the materials used to make candles, typically hydrocarbon waxes derived from petroleum or animals. The most common types include paraffin wax, beeswax, soy wax, palm wax, and gel wax. When heated, these waxes melt into a liquid state and can be poured into molds to create candles. An important property of candle waxes is their melting point and boiling point, which indicate the temperatures at which they transition between solid and liquid states. This determines how candles burn and allows candle makers to select waxes suitable for different applications.
Chemical Composition
The main types of wax used in candle making include paraffin wax, stearic acid, palm wax, and beeswax. Each has a slightly different chemical composition:
Paraffin wax is a byproduct of petroleum refining and is primarily composed of straight chain alkanes such as octadecane and nonadecane, with carbon chain lengths ranging from C20 to C40. Its chemical formula is CnH2n+2 where n is the number of carbon atoms (Elements of a Candle: Wax).
Stearic acid is a saturated fatty acid with an 18 carbon chain and chemical formula C17H35COOH. It is commonly combined with paraffin wax to raise the melting point and improve the candle’s burning properties (What is Candle Wax made of Chemically?).
Palm wax is derived from the palm oil plant and consists mainly of palmitic and oleic acids. Its chemical composition varies but generally contains fatty acids with carbon chains C16 and C18.
Beeswax is secreted by honey bees and composed primarily of esters of fatty acids and alcohols. Key components include palmitate, palmitoleate, and oleate esters of long chain alcohols such as triacontanol.
Melting Point vs Boiling Point
The melting point and boiling point are two important physical properties used to characterize substances. The melting point is the temperature at which a solid transitions to a liquid state, while the boiling point is the temperature at which a liquid transitions to a gaseous state.
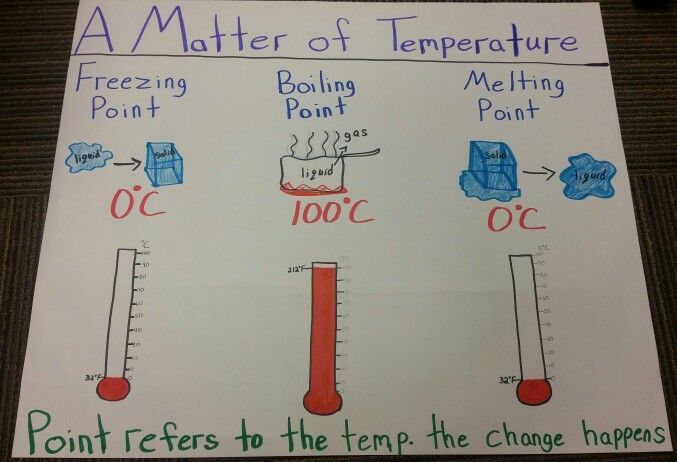
The key differences between melting point and boiling point are:
- State Change – Melting points indicate a solid to liquid transition, while boiling points indicate a liquid to gas transition.
- Temperature – Boiling points are always higher than melting points for a given substance. Water, for example, has a melting point of 0°C and a boiling point of 100°C.
- Intermolecular Forces – Stronger intermolecular forces (e.g. hydrogen bonding) lead to higher melting and boiling points.
- Kinetic Energy – More kinetic energy is required to overcome intermolecular forces to achieve the gaseous state at the boiling point compared to the liquid state at the melting point.
- Pressure – Unlike melting points, boiling points depend on ambient pressure. Higher pressures increase boiling points.
Understanding the differences between melting point and boiling point allows scientists to characterize and identify substances based on their physical properties.
Typical Boiling Points
The boiling point of candle wax can vary depending on the type of wax used. Candle wax is typically made from paraffin wax, beeswax, soy wax, or palm wax. Each type of wax has a different chemical composition, resulting in varying melting and boiling points.
According to Wax and Wick (source), paraffin wax has a boiling point range of 350-370°F (177-188°C). Beeswax boils at a higher temperature of 400-415°F (204-213°C). Soy wax has the lowest boiling point at 495-500°F (257-260°C). Palm wax boils around 500-525°F (260-274°C).
In summary, common candle waxes like paraffin, beeswax, soy, and palm wax boil in the range of 350-525°F (177-274°C). Paraffin has the lowest boiling point while palm wax has the highest. The exact boiling point depends on the specific chemical composition of each wax. When making candles, it’s important to know the boiling point range to avoid overheating the wax.
Measuring Boiling Points
There are several methods and laboratory equipment used to accurately measure the boiling point of a liquid substance. One of the most common methods is the capillary tube method. In this technique, a thin glass capillary tube is immersed in the heated liquid sample. The tube is observed closely as the liquid is heated. The temperature at which bubbles begin to emerge rapidly from the bottom of the tube is noted as the boiling point.
Other equipment such as a distillation apparatus or Thiele tube can also be used. These allow the vapors to be condensed and collected while monitoring the boiling point. Special ebulliometers are also designed to visually detect the boiling point through the tube walls or via thermometers. Regardless of equipment, accurate temperature measurement and close observation are critical in determining the definitive boiling point.
Factors Affecting Boiling Point
There are several key factors that affect the boiling point of a substance:
Molecular Weight – The boiling point tends to increase as the molecular weight of the substance increases. This is because larger molecules have more electrons and nuclei that interact, creating stronger intermolecular forces that require more energy to overcome (source 1).
Atmospheric Pressure – The boiling point of a liquid is the temperature at which its vapor pressure equals the environmental pressure surrounding the liquid. Therefore, the boiling point increases as external pressure increases. At higher external pressures, the liquid needs to acquire more kinetic energy for its vapor pressure to reach the external pressure, and thus reach its boiling point (source 2).
Impurities – The presence of impurities disrupts the intermolecular forces between molecules, making it easier for molecules to escape the liquid phase. This leads to a lower boiling point than for the pure substance (source 3).
Uses and Applications
Candle wax has a variety of uses and applications beyond just candle making. Some of the most common uses are:
Candle Making. Leftover candle wax is often reused to make new candles. The wax can be remelted and poured into containers or molds to create candles of any shape and size. Adding fragrance oils or dye allows for customized scented or colored candles. Reusing old wax is a crafty way to upcycle instead of throwing away.
Cosmetics. Beeswax and soy wax are sometimes used as ingredients in cosmetics like lip balms and lotions. The emollient wax helps condition skin and gives the products thickness and texture. Candle wax may also be incorporated into homemade beauty products like soaps and skin creams.
Art. Melted candle wax can be used for arts and crafts. It can be poured into molds to make decorative wax shapes. Wax is also used in batik, a fabric dying technique that involves applying wax to parts of the fabric before dying. The wax resists the dye and creates patterns and textures.
With some creativity, leftover candle wax has many possibilities for reuse and repurposing.
Interesting Facts
Candle wax has some interesting chemical properties that affect its melting and boiling points. According to https://candles.org/elements-of-a-candle/wax/, prior to the 19th century, most “wax” candles were made from beeswax. Beeswax has a relatively high melting point of 144-147°F due to its complex chemical composition.
Modern candle waxes are primarily made from paraffin, a petroleum byproduct. Paraffin has a lower melting point range of 115-150°F depending on the length of the hydrocarbon chains it contains. According to https://fraendi.com/blogs/blog/15-candle-facts-you-didnt-know-about, paraffin became popular because it was cheaper than animal-based waxes.
An interesting chemical property of waxes is that they don’t have a fixed boiling point. Instead, they gradually vaporize over a temperature range. The maximum boiling point depends on the wax’s chemical composition. Beeswax boils between 370-415°F, while paraffin boils between 350-550°F.
Safety Considerations
When working with hot wax, there are some important safety considerations to keep in mind:
Flammability
Candle wax can be extremely flammable, especially once it reaches its boiling point. The flash point of paraffin wax is around 204°C (399°F), which means it can ignite into flames at temperatures above this point (source). Proper precautions should be taken, such as avoiding open flames and other ignition sources when melting wax to higher temperatures.
Skin Irritation
Hot melted candle wax can cause burns and irritation when it comes in contact with skin. The wax itself is not toxic, but the high temperatures can damage skin tissue (source). Protective equipment like gloves and long sleeves should be worn when handling hot wax. If wax does spill on skin, run cool water over the area – do not try to peel solidified wax off.
In summary, candle wax can be safely handled if proper precautions around flammability and skin protection are taken.
Conclusion
The boiling point of candle wax provides significant insight into its properties and applications. Key takeaways include:
- Candle wax is composed primarily of hydrocarbons like paraffin, which have boiling points around 300-370°C.
- Measuring wax’s boiling point helps determine its melting behavior and ability to hold a wick and fragrance.
- Higher boiling points indicate more complex hydrocarbons that are slower to vaporize and melt.
- Additives like dyes and scents can slightly alter the boiling point of finished candle wax.
- Understanding wax’s boiling point assists in designing safe, high-performing candle products.
In summary, the boiling point provides a critical scientific parameter for qualifying and formulating candle wax. This knowledge empowers effective and safe candle making across industrial and hobbyist settings.






