What Happens To Wax When Candle Burns?
Wax is a natural substance that is created through various chemical processes. It has served a range of important purposes across human history, with one of the most notable uses being candle making.[ 1 ]
Wax refers to a class of organic compounds that are malleable solids at ambient temperatures. It is characterized by being plastic when warm, and tending to melt at higher temperatures rather than burning or evaporating. Wax exhibits a low viscosity when melted.[ 2 ]
Due to its unique properties, wax has been utilized by humans for thousands of years. Some of the earliest evidence of wax candle making dates back to Ancient Egypt around 3,000 BC. Candles provided an important source of light before the invention of electric lighting. Wax was also used for sealing letters, waterproofing surfaces, and creating molds.[ 3 ]
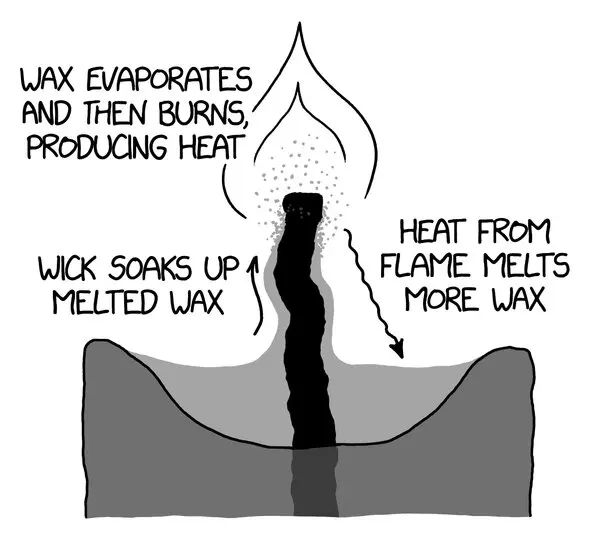
Over time, various types of wax have been used for candle making including beeswax, whale fat, tallow, and paraffin wax. The unique properties of wax make it ideal for candle creation. When lit, the wax melts and is drawn up the wick where it vaporizes, helping sustain the flame.
This article will explore what happens chemically and physically when a wax candle burns. We will examine the components of candles, wax properties, the combustion process, and factors that influence how a candle burns.
[1] https://candles.org/history/
[2] https://www.britannica.com/science/wax-chemical-compound
[3] https://creativecandles.com/blogs/blog/a-history-of-candle-use
Chemical Composition of Wax
The main types of wax used in candle making are paraffin wax, beeswax, soy wax, palm wax, and gels. Paraffin wax is a byproduct of petroleum refining and is composed of straight chain alkanes such as octacosane and hexatriacontane. Beeswax is produced by honey bees and contains fatty acid esters, hydroxyl fatty acids, and hydrocarbons. Soy wax is made from hydrogenated soybean oil and has a high content of triglycerides. Palm wax comes from the leaves of palm trees and contains fatty acids, alcohols, hydrocarbons, and esters. Gels used in jar candles can be composed of mineral oil, resins, polymers, or plant-based oils.
The chemical composition of the different waxes impacts the burning properties of the candle. Paraffin wax has a relatively low melting point, allowing it to liquefy into fuel for the candle flame. Beeswax has some naturally occurring rubber-like polymers that increase its rigidity. Soy wax contains fatty acids that help anchor fragrances. The type of wax impacts characteristics like melting point, surface adhesion, rigidity, opacity, and ability to hold fragrance.
Sources:
https://www.scribd.com/document/653186864/Cooking-Oil-as-an-Alternative-Candle
https://cs.veganapati.pt/what-is-chemical-composition-candle-wax
Wick in Candles
The wick is an essential component in candle making and burning. The main purpose of the wick is to deliver fuel to the flame and allow the wax to burn in a controlled way (CitizenSide). Without a wick, the candle wax would simply melt into a puddle of liquid.
The wick works by capillary action. As the melted wax gets absorbed into the wick, it acts as a fuel to keep the flame going. The wick also regulates the melting rate and size of the flame. Thicker wicks allow more wax to travel up, resulting in a larger flame and faster burning. Thinner wicks have the opposite effect.
Wicks are typically made from braided cotton or paper fibers. Cotton provides a nice balance of flexibility and stiffness to draw the liquid wax up. Some specialty wicks may contain zinc or tin cores to help maintain their shape as they burn. Proper wicking is crucial for even burning, wax pooling, and preventing issues like tunneling.
Melting Point of Wax
The melting point of wax refers to the temperature at which solid wax transitions to a liquid state. This is an important property of candle wax because the wax needs to be heated to a liquid in order to be poured into candle molds and to allow for capillary action as the candle burns.
Different types of candle waxes have different melting points. According to the Melting Point Factors for Common Waxes article, paraffin wax generally has a melting point between 47–65°C (116-149°F) depending on oil content. Beeswax has a melting point of 62-65°C (143-148°F). Soy wax melts at 55-60°C (130-140°F). Palm wax melts around 62-65°C (143-148°F) https://blendedwaxes.com/blog/wax-melting-point-factors/.
Candle waxes with higher melting points are harder, retain their shape better in warm environments, and burn longer. Lower melting point waxes burn at lower temperatures but can lose their structural integrity easier. Matching the melting point with the desired candle performance is an important consideration.
Capillary Action
Capillary action is the phenomena that allows the melted wax to travel up the wick of a burning candle. It occurs due to the interaction between the liquid wax, the solid wick, and the surrounding air.
As the flame melts the wax at the tip of the wick, the liquid wax molecules are attracted to each other (cohesion) and to the molecules of the porous wick (adhesion). This capillary action creates an upward force that draws the liquid wax into the wick fibers against gravity.
The smaller the diameter of the wick, the farther the liquid can travel upwards due to the increased ratio of adhesive to cohesive forces. The liquid wax travels up until it reaches an equilibrium point or the top of the wick.
The wax then vaporizes at the top of the wick where it meets the flame. This capillary action process continuously transports more liquid wax to fuel the candle as it burns.
(Source: https://www.cbsnews.com/pittsburgh/news/hey-ray-candles-and-capillary-action/)
Vaporization and Combustion
As the candle wax melts, it begins to vaporize into a gaseous state. This vaporization occurs before the wax reaches its boiling point temperature. The wax vapors then travel up the wick through capillary action. When the vapors reach the tip of the wick, they mix with oxygen in the air and ignite, resulting in a flame.
The combustion reaction occurs between the vaporized wax molecules and oxygen gas. As they combine, energy is released in the form of light and heat. This exothermic reaction sustains the flame as long as there is melted wax feeding the wick to produce more vapor.
According to a flow visualization report, the phase change from solid wax to vapor highlights the vaporization process occurring in a burning candle.
Products of Burning
When a candle burns, it undergoes a chemical reaction that breaks down the waxes (hydrocarbons) into different products. The main products of a candle burning are:
- Carbon dioxide (CO2) – Candles produce carbon dioxide as a result of the reaction between carbon in the waxes and oxygen in the air. This is the same gas that humans and animals exhale.
- Water vapor (H2O) – The hydrogen in the waxes reacts with oxygen to produce water, which evaporates as steam. This is a major component of the hot wax vapors emitted from the melting wax pool.
- Soot – Incomplete combustion can result in soot, which is composed of unburned carbon particles. This black residue collects on candle holders and surfaces.
The chemical reaction is essentially:
Hydrocarbons (waxes) + Oxygen → Carbon dioxide + Water + Heat/Light Energy (+ trace amounts of soot)
The main desired products of a burning candle are the light and heat energy. However, carbon dioxide and water vapor are unavoidable byproducts. Properly formulated candles with the right wick and wax will burn cleaner and produce less soot.
(Source: https://candles.org/candle-science/)
Candle Flame Structure
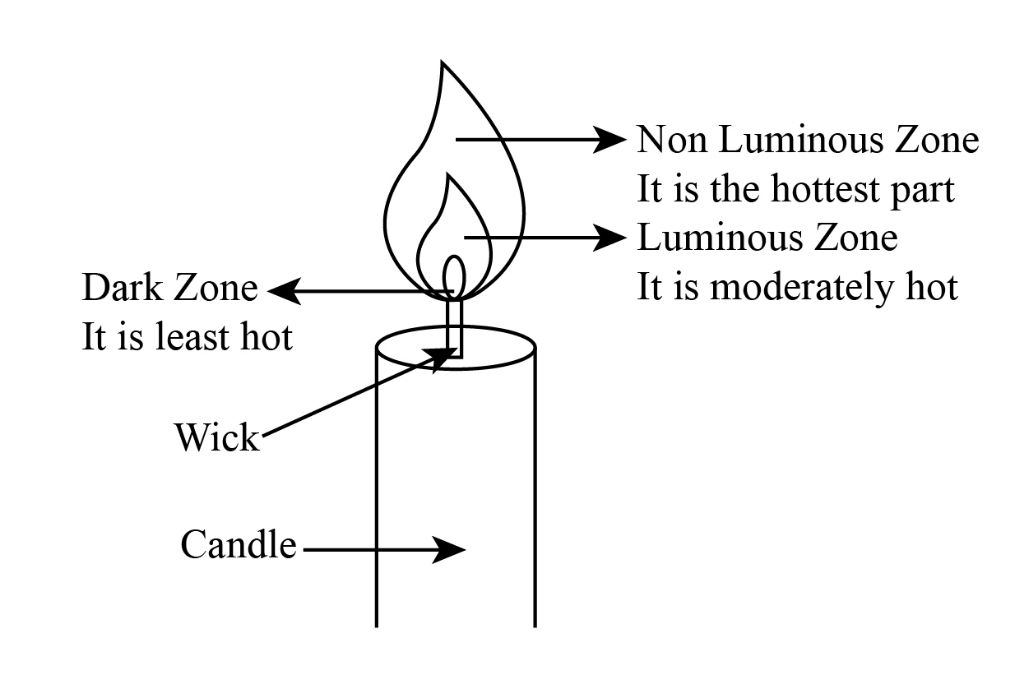
The flame of a candle has a distinct structure with several identifiable parts. At the base of the flame is the wick, which absorbs liquefied wax via capillary action and brings it to the flame. Surrounding the wick is the inner zone, which is where the wax vaporizes. This zone does not emit much light.
Just above the inner zone is the inner mantle, which is the hottest part of the flame. This is where complete combustion of the wax vapor occurs. Soot particles in the wick ignite and emit a bright yellow light in this zone. The temperature can reach around 1400°C.
Above the inner mantle is the outer mantle, where incomplete combustion produces small soot particles that glow orange. The outer mantle does not burn as hot as the inner mantle.
The outermost part of the flame is the halo or outer zone. This is a translucent region of heated air and combustion products like water vapor and carbon dioxide. The halo is the coolest zone in the flame.
Hot gases from combustion rise up through the flame. As they move upwards, cooler gases flow downwards along the sides in a convection current, bringing fresh oxygen to the flame.
The distinct zones of the candle flame result from differences in temperature and the complex chemistry occurring during the combustion of wax.
For more details on candle flame anatomy, check out this informative video: https://www.tiktok.com/discover/science-of-candle-flame
Environmental Factors
The environment around a burning candle can significantly impact how it burns. Temperature is one of the biggest factors. In colder environments, the wax cools faster, which can cause the candle to tunnel or form a cupped top as the wax solidifies before reaching the edges. Temperature also impacts wax viscosity – in warmer environments, the melted wax is thinner and can flow more easily up the wick. Source
Air currents and drafts are another environmental factor. The flickering flame of a candle needs still air surrounding it to burn properly. Drafts can cause the flame to flicker excessively, tunnel, smoke, produce excessive soot, and in severe cases, blow out. This is why candles often include recommendations to avoid drafty areas. Shielding candles from drafts, such as inside glass containers, can improve performance. Source
The stability and flatness of the candle’s base also impacts burning performance. On an uneven surface, the wax pool will not heat evenly, causing issues like tunneling. The candle holder material is also a factor – materials like metal and glass conduct heat away from the base, while insulating bases allow for better wax pool formation.
Conclusion
In summary, understanding the science behind how candles burn provides insight into chemistry, physics, and everyday objects. When a candle burns, the solid wax melts and is drawn up the wick via capillary action. The liquid wax vaporizes at the flame and undergoes combustion reactions with oxygen to produce heat, light, carbon dioxide, and water vapor. The shape and behavior of the flame is influenced by convection currents, air pressure, and other environmental factors. Knowing the mechanisms involved in candle burning allows people to optimize burn time, fragrance, and safety. Overall, investigating something as common as a burning candle reveals a fascinating interplay of scientific principles.
Gaining a deeper knowledge of how candles work demonstrates that even simple, everyday objects operate based on complex chemical and physical processes. Examining candle science encourages curiosity about the world and appreciation for the underlying order and logic governing seemingly mundane things. In addition, understanding the science behind objects we use daily could inspire future scientists and inventors to make discoveries that improve people’s lives.