What Are Waxes Formed From?
Waxes are a diverse class of organic compounds that are lipophilic, malleable solids at around ambient temperatures. They consist of long-chain aliphatic hydrocarbons (alkanes), alcohols, fatty acids, and esters that are insoluble in water (Source). Chemically, waxes are esters of long chain fatty acids and long chain alcohols. The fatty acids often contain 12-32 carbons while the alcohols have 20-40 carbon atoms (Source).
This article will provide an overview of the main sources of waxes, including plant waxes, animal waxes, petroleum waxes, mineral waxes, and synthetic waxes. It will examine the chemical composition, properties, and uses of the major types of waxes derived from these sources.
Plant Waxes
Plant surfaces are covered with a cuticle, which is composed of cuticular waxes. These waxes form a protective layer that waterproofs the aboveground parts of plants. The main components of cuticular waxes are very-long-chain lipids and their derivatives, like fatty acids, primary and secondary alcohols, aldehydes, alkanes, esters, and cyclic compounds.
The chemical composition and structure of cuticular waxes play an important role in minimizing water loss, regulating transpiration, and providing insect and pathogen resistance. The crystalline ultrastructure of the wax film enables the formation of a hydrophobic barrier against external water. The composition of cuticular waxes varies between plant species, tissues, developmental stages, and in response to environmental conditions. Modifying the wax composition can help plants adapt to drought, high temperature, and other stresses.
Some key references on plant waxes:
https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2022.888930/full
https://pubmed.ncbi.nlm.nih.gov/35874003/
Animal Waxes
Beeswax is produced by honey bees in their hives. Bees consume honey and secrete wax from glands on their abdomens. They use the wax to build the familiar hexagonal honeycomb structures to store honey and eggs. The wax is harvested by beekeepers along with the honey by melting the wax off the honeycombs. Beeswax consists primarily of esters of fatty acids and various long chain alcohols. It also contains some free fatty acids, resinous compounds, and proteinaceous material. References: https://bzzwax.com/blogs/all-beeswax/what-is-beeswax-and-how-its-made, https://bestbees.com/2022/11/30/uses-for-beeswax/
Lanolin is a wax secreted from the sebaceous glands of wool-bearing animals like sheep. It is extracted from raw wool during processing and purified into a thick yellow wax. Lanolin consists primarily of sterol esters of high molecular weight alcohols and fatty acids. It also contains some lanosterol, cholesterol, and fatty alcohols. Lanolin has many uses, including lubricants, moisturizers, and corrosion protectants. Reference: https://beeswax.co.nz/beeswax-information/
Petroleum Waxes
Petroleum waxes are byproducts of petroleum refining. The two main types of petroleum waxes are paraffin wax and microcrystalline wax.
Paraffin wax is separated from the heavier fractions of crude oil during the refining process. The process involves extracting the wax using a crystallization technique called solvent dewaxing. The wax crystals are then refined through hydrofinishing to improve color and odor. Paraffin waxes are composed of straight chain alkanes with 20 to 40 carbon atoms (https://www.petronaftco.com/paraffin-wax-production-process/).
Microcrystalline waxes are extracted from the heavy residual fractions of crude oil. They undergo a more extensive refining process called catalytic dewaxing to remove aromatics and achieve the desired properties. Microcrystalline waxes contain branched and cyclic alkanes and have smaller crystal size compared to paraffin (http://www.amwax.net/Wax_abc2.asp).
Mineral Waxes
Montan wax comes from lignite or coal deposits and is the most common mineral wax. The composition of montan wax varies based on the plant material that was fossilized and the conditions used during solvent extraction.[1] However, the key ingredients are:
- Esters of high molecular weight alcohols and high molecular weight fatty acids
- Free long chain acids
- Long chain alcohols
- Hydrocarbons

The esters provide hardness and high melting points, while the other components help provide plasticity. Typical properties of montan wax include a melting point around 82–95°C, low viscosity when melted, and water repellency. Montan waxes from different deposits can vary in color from dark brown to light yellow.[2]
Montan waxes are widely used in leather dressing, cosmetics, polishes, electrical insulation, and lubricants. The montan wax composition makes it well-suited for these applications that require water repellency combined with lubricity.
[1] https://www.waxemulsion.info/what-is-wax/
[2] https://patents.google.com/patent/US4398953A/en
Synthetic Waxes
There are several types of synthetic waxes produced from chemical reactions and polymerization processes. The most commonly produced synthetic waxes are Fischer-Tropsch waxes and polyethylene waxes.
Fischer-Tropsch waxes are produced by the Fischer-Tropsch process which converts carbon monoxide and hydrogen into liquid hydrocarbons. This process allows the production of waxes with customized properties by controlling the reaction conditions and catalysts. These waxes are primarily composed of normal paraffins with very low aromatic content (Discover Three Types of Synthetic Wax).
Polyethylene waxes are created through the polymerization of ethylene gas. By controlling the polymerization process, waxes can be produced with different properties like melting point, hardness, and viscosity. Polyethylene waxes have a wide range of applications including plastic processing, inks, coatings, and polishes (Synthetic waxes – Polyethylene Wax Refining).
Other types of synthetic waxes include polypropylene waxes produced from propylene polymerization and synthetic paraffin waxes produced from purified petroleum feedstocks. Compared to natural waxes, synthetic waxes can be customized to have specific properties and consistent chemical compositions.
Wax Modifications
Waxes can be chemically modified to adjust their properties and meet specific market needs. Common wax modifications include hydrogenation, oxidation, and emulsification.
Hydrogenation involves reacting the wax with hydrogen gas in the presence of a catalyst. This saturates double bonds in the wax molecules, increasing the melting point and improving hardness and gloss. Hydrogenated waxes are widely used in cosmetics, pharmaceuticals, and food coatings [1].
Oxidation introduces oxygen molecules into the wax, making it more polar and water-dispersible. Oxidized waxes can be emulsified in water for applications like wax coatings for fruit and paper. Trecora’s OX7 and OX18 are examples of oxidized waxes used in emulsions [2].
Emulsification allows waxes to be dispersed in water by using surfactants and high-shear mixing. Wax emulsions provide moisture barrier coatings on fruits, vegetables, and paper products. They are also used in polishes and release agents [3].
Major Wax Types
Some of the most common and commercially significant types of wax include:
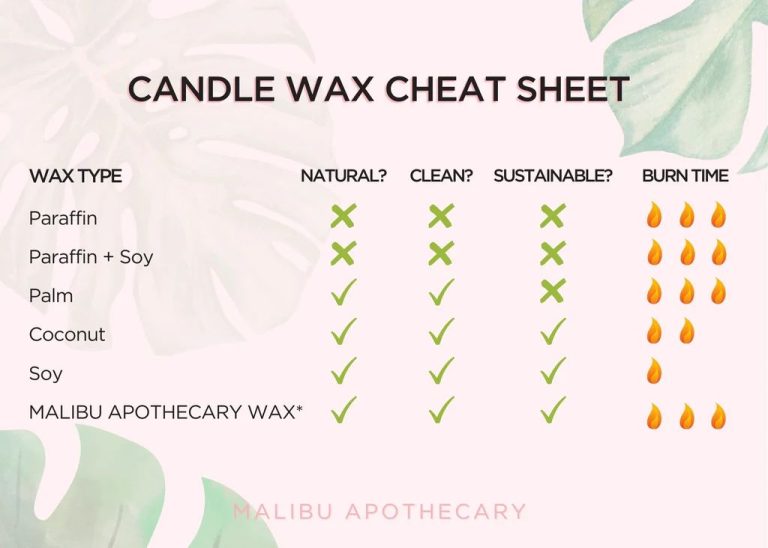
Paraffin wax is one of the most widely used waxes. It is extracted from petroleum and coal during the refining process. Paraffin wax is odorless and colorless, with a melting point between 47°C and 64°C. It’s commonly used in candles, cosmetics, polishes, and lubricants. Paraffin is hard, durable, and inexpensive, making it a popular choice for commercial applications.1
Beeswax is a natural wax made by honey bees. It has a mild, honey-like scent and is yellow in color. Beeswax has a relatively low melting point of 62°C to 64°C. It’s used in a variety of products like candles, lip balms, creams, and as a food additive. Beeswax is valued for being all-natural, non-toxic, and compatible with skin.2
Carnauba wax comes from the leaves of the carnauba palm tree. It is very hard with a high melting point between 82°C and 86°C. Carnauba wax is used to strengthen and harden products like candles, candy, dental floss, and automobile waxes. It produces a glossy shine and water-repellant coating.2
These major wax types each have unique properties that make them suitable for different applications. Beeswax and carnauba wax provide natural options, while paraffin wax is ideal for large-scale commercial uses. Waxes help create durable, shiny, and water-resistant products across many industries.
Wax Industry
The global wax market size was valued at USD 9.7 billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 3.6% from 2022 to 2030 (Global Car Wax Industry Share and 2021 Forecast Analysis Report). Major producers of wax include companies like ExxonMobil, Shell, PetroChina Company Limited, Lukoil, and BP (Emulsifying Wax Market Research: Global Status & Forecast).
Key drivers for the wax market include growing demand from applications like candles, packaging, cosmetics, and polishes/coatings. The increasing use of wax in the adhesive industry is also driving growth as waxes are used to modify viscosity, enhance moisture resistance, and improve other properties of adhesives (Global Candelilla Wax Industry Market Report 2020).
Asia Pacific accounted for the largest share of the global wax market in 2021. This was attributed to rapid industrialization and growing demand from key countries like China, India, Japan, and South Korea. North America and Europe are mature markets expected to grow steadily. The Middle East & Africa and South America offer lucrative opportunities for market expansion due to increasing infrastructure activities in these regions.
Conclusion
In summary, waxes come from a variety of natural and synthetic sources. Key wax sources include plants, animals, petroleum, and minerals. Beeswax and carnauba wax are two of the most widely used natural waxes, derived from honeycomb and palm leaves respectively. Paraffin wax is a major petroleum wax, while montan wax comes from lignite coal. Synthetic waxes such as polyethylene and Fischer-Tropsch wax offer uniform properties.
Waxes play an important commercial and industrial role due to their unique properties. They are used for candles, cosmetics, polishes, electrical insulation, and food packaging. The versatility of waxes allows them to provide waterproofing, lubrication, shine, and texture across many applications. Global demand for waxes is increasing, especially in emerging markets, leading to industry growth. Waxes improve many manufacturing processes and commercial products that consumers rely on every day.