How Does Candle Wax Disappear?
Candle wax is typically composed of paraffin wax, a common byproduct of petroleum refining (https://patents.google.com/patent/US20170247634A1/en). It has a low melting point which allows it to transition from a solid to a liquid state as the candle burns. The disappearance of candle wax is a complex process that involves the wax melting due to heat, wicking along the candle wick, vaporizing into the air, and the vapors combusting to form carbon dioxide and water. This results in the wax “disappearing” as it undergoes phase changes from solid to liquid to gas.
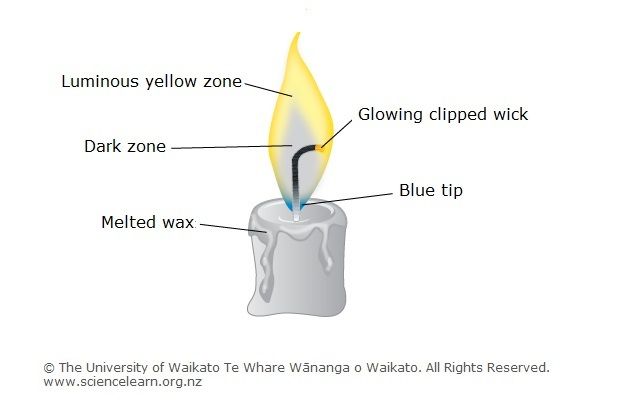
Wick
The wick is a key component that enables candles to burn. As the National Candle Association explains, “A candle wick works like a straw to deliver the fuel to the flame. It transports melted wax to the flame. When lit, the wick produces the flame. The capillary action of the wick draws the melted wax upwards aiding to fuel the flame” [1]. The wick contains tiny fibers that absorb and transport the melted wax via capillary action.
Wicks are traditionally made from materials like cotton, linen, or paper that easily soak up wax. More modern wicks may contain materials like zinc or tin to help prevent issues like mushrooming. The thickness and braiding of the wick impacts how quickly wax can travel up to the flame. Thicker wicks allow more wax to burn at once, creating a larger flame. Carefully pairing the wick type to the wax helps ensure an optimal burn.
Melting Point
The melting point of candle wax is an important factor in how candles burn. Paraffin wax, the most common candle wax, has a melting point between 130-150°F according to this source. Soy wax, another popular candle wax, melts at temperatures between 115-135°F. The melting point determines the ideal temperature for adding fragrance oils and pouring wax into containers.
Wax needs to be heated approximately 20 degrees above its melting point before adding fragrance oils. If the wax is too cool, the oils won’t properly bind with the wax. If the wax is too hot, the fragrance may burn off. Most candle fragrances are formulated to withstand temperatures up to 170°F before breaking down. After adding fragrance, the wax can be poured once it has cooled to approximately 10 degrees above the melting point.
The melting point is why the wax closest to the wick melts and travels up through the wick via capillary action. The melted wax is then vaporized by the flame to release the scent throw. The wax further from the flame remains solid until it heats up enough to melt and continue fueling the candle as it burns down.
Capillary Action
Capillary action refers to the ability of a liquid to flow against gravity in narrow spaces such as small tubes, pores, or spaces between fibers. This phenomenon is key to understanding how candle wax “disappears” as the candle burns.
Candle wicks are typically made of braided cotton fibers. These fibers have small gaps between them that allow liquid wax to be drawn up the wick via capillary action. The wax liquifies due to the heat of the flame and is pulled up the wick. As the wax reaches the flame, it then vaporizes and burns.
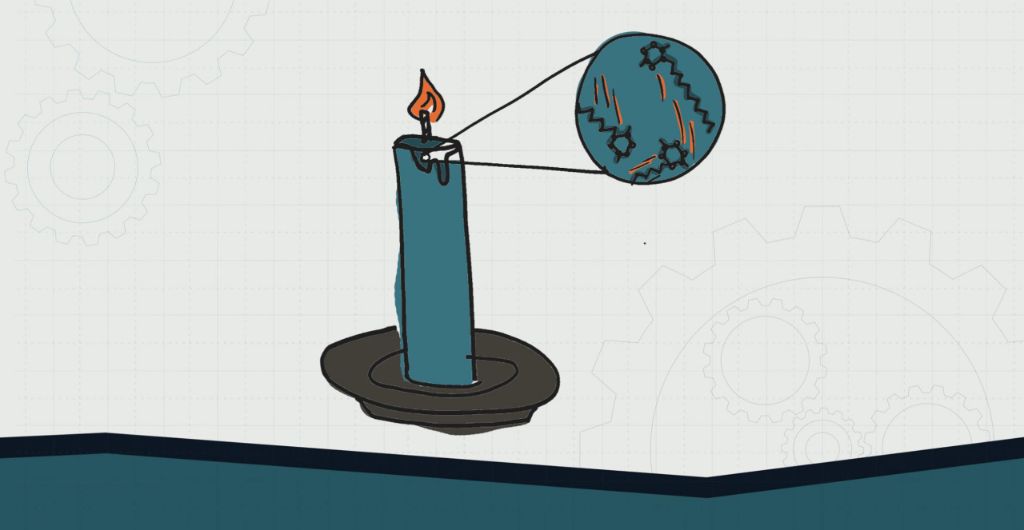
Capillary action occurs due to an interplay between the adhesive intermolecular forces between the liquid and the surrounding solid surface, and the cohesive intermolecular forces within the liquid itself. The adhesive forces cause the liquid to cling to the surface and move up, while the cohesive forces give the liquid integrity and prevent it from separating.[1]
Therefore, the narrow spaces within the cotton wick fibers create ideal conditions for capillary action to take place and draw the melted wax upwards as the candle burns.
Vaporization
As the flame burns, it provides heat that is conducted down the wick to the liquid wax pool. This heat causes the liquid wax to vaporize, or turn from a liquid into a gas. According to Candle Science, “The heat of the flame vaporizes the liquid wax (turns it into a hot gas), and starts to break down the wax vapor into particles small enough to get oxygen and continue the flame.”1 The wax needs to vaporize before it can mix with oxygen and burn. Vaporization allows the wax molecules to spread out and mix with oxygen, enabling combustion.
The vaporization of wax is a key part of how candles work. The heat must be sufficient to change the state of wax from liquid to gas for the wax to burn. This explains why the wax near the wick burns, while solid wax on the sides and bottom of a candle jar does not – the heat from the flame only reaches high enough temperatures near the wick to cause vaporization.
Convection Currents
As the flame heats the air above it, convection currents are created. Convection currents refer to the circular motion of fluids and gases due to differences in temperature and density. In the case of a candle, the hot air right above the flame becomes less dense than the surrounding cooler air. As a result, the hot air rises while the surrounding cooler air sinks to take its place. This creates a convection current, drawing oxygen downward to feed the flame and releasing carbon dioxide upward.
This convection current is critical for allowing the candle wax to vaporize and the wick to keep burning. As hot air rises, it pulls in new oxygen from the sides to sustain combustion at the wick. This incoming oxygen also evaporates the wax into a vapor that can be burned. Without these convection currents continuously bringing fresh oxygen, the flame would quickly use up the available oxygen and be snuffed out.
Experiments using a box with two vertical chimneys and a candle demonstrate these convection currents well. When a candle is lit at the bottom, smoke clearly moves downward in one chimney as fresh air is drawn into the box. The smoke then flows up and out the other chimney as the hot air rises after being heated by the flame. This cycle repeats itself continually, powering the combustion of the candle wax and wick.(Chimney Convection Box, n.d.)
Combustion
When a candle burns, the candle wax undergoes combustion. Combustion is a chemical reaction between a fuel and an oxidant, such as oxygen gas. In the case of a burning candle, the wax acts as the fuel source while the oxygen in the air acts as the oxidant.
As the candle wax melts from the heat of the flame, it is drawn up through the wick via capillary action. The liquefied wax then vaporizes due to the high temperatures at the top of the wick. This wax vapor combines with oxygen in the flame, initiating the combustion reaction.
The main chemical reaction occurring is:
C25H52 + 38 O2 → 25 CO2 + 26 H2O
The hydrocarbon compounds in the wax (C25H52) combine with oxygen gas (O2), producing carbon dioxide (CO2) and water vapor (H2O) as waste products. The energy released provides the heat and light of the flame. Therefore, the wax vaporizes and reacts with oxygen in the combustion process, disappearing over time.
Carbon Dioxide + Water
When a candle burns, the flame produces carbon dioxide (CO2) and water vapor as byproducts of combustion. As the candle wax melts and vaporizes, its hydrocarbon molecules combine with oxygen in the air to produce CO2 and H2O.
Specifically, the paraffin wax used in most candles contains long chains of carbon and hydrogen atoms. During combustion, the carbon-to-carbon bonds are broken, and the carbon atoms combine with oxygen to form CO2. The hydrogen atoms combine with oxygen to create H2O vapor.
According to research from the National Candlemaker Association, a candle burning for one hour will release approximately 2.6 grams of CO2. While not a significant source compared to other combustion processes, the CO2 emissions from burning candles do contribute to the greenhouse effect.
The water vapor released is not harmful and simply dissipates into the air. The amount of water produced is approximately 1.5 grams per hour of candle burning. This moisture can sometimes be seen when burning a candle in a cold environment, as the water vapor condenses into water droplets.
So in summary, the CO2 and H2O are normal byproducts of the chemical reaction that allows candles to burn and release light and fragrance. The CO2 contributes slightly to global CO2 emissions, while the water vapor is insignificant and harmless.
Dispersal
As a candle burns, the main gaseous byproducts produced are carbon dioxide and water vapor. These gases quickly disperse and mix with the surrounding air. According to the Candle Science website, “These vaporized molecules are drawn up into the flame, where they react with oxygen from the air to create heat, light, water vapor (H2O) and carbon dioxide (CO2).”
The carbon dioxide and water vapor gases are less dense than the rest of the air, so they rise up into the air surrounding the flame. Convection currents also help circulate the gases, dispersing them into the room.
Within minutes, the gases are diluted as they mix with the ambient air. The carbon dioxide and water vapor spread out, reaching an equilibrium concentration harmless to humans. This rapid dispersal into the large volume of room air prevents a dangerous buildup of the gases produced by the candle.
Conclusion
In summary, the disappearance of candle wax is a multi-step process that fully converts solid wax into gaseous products through melting, vaporization, and combustion. As the candle burns, the solid wax melts and is drawn up the wick by capillary action. The melted wax reaches the flame where it vaporizes into a hot wax vapor. This vaporized wax then undergoes combustion reactions with oxygen to produce carbon dioxide and water vapor. These gaseous products rise into the air through convection currents and disperse, completing the conversion of solid candle wax into gases.
The wax does not stay behind in liquid or solid form, but is instead fully converted to gases that rise and spread into the surrounding air. This comprehensive process explains how and why candle wax seems to completely disappear as it burns.