Does The Candle In A Jar Evaporate?
Candles in jars have become popular decorative and fragrance items. A unique aspect of candles in jars is how the candle interacts with the jar as it burns. Specifically, many people wonder what happens to the wax as the candle burns down in the jar over time. Does the wax evaporate like the wax burning on the wick? This article will examine the science behind candle evaporation and whether candles in jars fully evaporate.
We’ll start by overviewing the process of evaporation and how it applies to candle wax. Next, we’ll discuss specific factors that affect evaporation rates of candles in jars, like jar material, size, candle composition, etc. To understand why wax may build up, we’ll look at the chemistry of burning candles. Finally, we’ll cover maintenance tips for handling wax buildup in candle jars and key safety considerations.
What is Evaporation?
Evaporation is the process through which a liquid substance transitions into a gaseous state below its boiling point (https://byjus.com/chemistry/evaporation-causes-cooling/). When a liquid is exposed to the atmosphere, molecules near the surface absorb enough energy to overcome intermolecular attractive forces and escape into the air as vapor. This transition from liquid to gas happens spontaneously at temperatures below the liquid’s boiling point. As the most energetic surface molecules leave, more liquid molecules rise to take their place and are in turn evaporated. This cycle continues as long as conditions allow evaporation to occur.
Some key aspects of the evaporation process are:
– Only the molecules at the surface of the liquid can evaporate, so increasing surface area speeds up the process.
– Heat or thermal energy is needed for evaporation to occur, as it gives molecules the energy needed to escape the liquid.
– Factors like temperature, humidity, wind speed and surface area affect the rate of evaporation, but not whether evaporation happens.
– Evaporation causes cooling as the highest energy molecules escape, leaving the remaining liquid with lower average energy (https://study.com/academy/lesson/what-is-evaporation-definition-examples-quiz.html).
In summary, evaporation is the process by which molecules spontaneously transition from liquid to gaseous state below the boiling point, requiring heat energy and causing a cooling effect on the remaining liquid.
Do Candles Evaporate?
Yes, candles do evaporate over time as the wax vaporizes. When a candle is lit, the flame melts the solid wax which is then drawn up the wick via capillary action. As the liquified wax reaches the flame, it vaporizes into a gas which provides the fuel for the flame. This process continues as long as there is solid wax for the flame to melt and turn into a vapor. According to the New York Times article “Where Does a Candle Go When It Burns?”, the light and heat from a burning candle comes from the wax undergoing combustion and evaporating into the air (https://www.nytimes.com/2021/04/26/science/randall-munroe-candle-xkcd.html).

Some of the wax does get left behind in the form of melted wax pooling around the wick. But over time as the candle continues to burn, the wax vaporizes faster than the melted wax gets replenished by the solid wax. This evaporation process continues until all the solid wax has been vaporized.
Candle Evaporation Science
The evaporation of a candle is driven by basic chemistry and physics principles. When a candle burns, the wick draws up melted wax via capillary action. The heat of the flame vaporizes the liquid wax and breaks down the hydrocarbon molecules into hydrogen and carbon atoms (https://candles.org/candle-science/).
As the hydrocarbon molecules break down, the atoms reform into new smaller molecules like methane, hydrogen, and carbon dioxide. This chemical breakdown releases energy in the form of light and heat. The vaporized wax molecules are carried upwards by convection and diffusion. When they reach the cooler air above the flame, the wax vapors condense into tiny airborne particles of unburned wax.
The condensation of wax decreases the number of air particles and reduces air pressure in the jar. This reduction in pressure draws up water to replace the missing air volume when the candle is extinguished (https://people.math.harvard.edu/~knill/pedagogy/waterexperiment/). The water displacement visually demonstrates the air pressure decrease from wax vapor condensation.
Evaporation Rate Factors
When a candle burns, the heat of the flame causes the wax to melt and evaporate into the air. However, the rate at which the wax evaporates depends on several factors. Here are some of the key things that affect the evaporation rate of candles:
Temperature – Higher temperatures increase the evaporation rate of the candle wax as it causes the wax molecules to move faster, allowing them to escape into the air at a quicker pace. Burning a candle in a warmer environment will make it evaporate faster than burning it in a colder setting.
Air Flow – Good air circulation around a burning candle can accelerate evaporation. As air currents remove evaporated wax molecules away from the flame, it allows more wax to evaporate. Burning a candle in an enclosed space with little air flow will slow down the evaporation rate.
Candle Ingredients – The specific waxes and other ingredients used in a candle affect how quickly it evaporates. Candles made from waxes with lower melting points, like paraffin, tend to evaporate faster than those made from waxes with higher melting points. Things like scented oils and additives can also influence evaporation rates.
So in summary, factors like higher temperatures, good air flow, and the type of wax used can increase the evaporation rate of a burning candle. Knowing how these elements impact evaporation allows you to better manage candle burn times.
Candles in Jars
When a candle is burned in a jar, the restricted air flow can impact evaporation and wax build up. As the candle burns, some of the wax turns from a solid to a gas through evaporation. However, since the jar restricts air flow, the gaseous wax has nowhere to go and condenses back into a solid on the sides of the jar.1 This causes a build up of wax on the interior sides of the jar over time.
Jars are attractive candle holders, but the restricted air flow means wax evaporates slower compared to an open candle. The wax build up can become extreme enough that it starts to drown the wick, preventing the candle from burning properly. This wax build up is the tradeoff for using cute jars.
Wax Build Up
Over time, the candle wax inside the jar will evaporate and leave behind residue on the inner sides of the jar. According to The Spruce, as the wax pool burns down, some wax molecules moving up through the wick may not fully combust and condense on the jar’s sides instead.
The wax buildup occurs because of the candle wax evaporating, leaving behind the non-combustible waxy components. The evaporation rate of candles in jars is generally slower than free-standing candles, as the jar helps insulate the candle and restricts air flow. But evaporation still inevitably occurs.
The wax left behind after evaporation can slowly build up on the glass with repeated burning. The wax buildup may appear as a white, opaque, or yellowish film. Over many burns, it can become quite thick and difficult to remove from the jar.
Thick wax residue can also begin to flake off into the melting wax pool. This can clog the wick as bits of wax drop down into the pool, disrupting capillary action. So it’s important to clean the buildup for optimal candle burning.
According to Architectural Digest, letting the jar sit in hot water can help soften built-up wax for easier removal (source). Using adhesive removers like Goo Gone can also help scrub off stubborn wax (source).
Maintaining Candles in Jars
When burning candles in jars, some evaporation and wax build up is inevitable. Here are some tips for managing these issues:
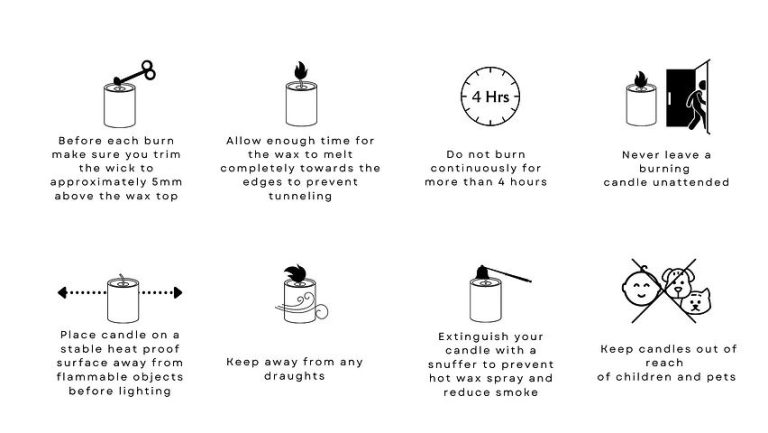
Trim the wick to 1⁄4 inch before lighting to prevent excess smoke and soot, which can dirty the jar and contribute to more buildup. Trim the wick before each use.
Pour out excess wax to limit buildup. Allow the candle to burn for 4-6 hours, blow it out, and let it cool completely. Carefully pour the melted wax into a heat safe container. Repeat this every 10-15 hours of burn time.
Wipe the inside of the jar while the wax is liquid to remove residue. Use paper towels dampened with a small amount of rubbing alcohol or vinegar. This helps limit cloudy wax build up over time.
Consider pouring smaller amounts of wax into the jar, around 1 inch deep, to allow more room for wax to pool while burning. This gives evaporated wax somewhere to go before hitting the sides.
Clean the jar thoroughly when empty by filling halfway with water and boiling on the stove until the wax melts. Drain the water and wipe away any wax residue. Allow to fully dry before refilling with a new candle.
Rotate multiple jars to give each one a break between uses. Allowing time for the glass to fully cool prevents wax from adhering as strongly.
Store candles with lids between uses to minimize dust and dirt exposure that can affect evaporation and lead to more buildup over time.
Consider using a candle warmer instead of an open flame to melt and release fragrance. This heating method minimizes evaporation and residue.
With proper care, trimming, pouring, cleaning, and rotation, candles in jars can provide enjoyment over many uses before requiring replacement.
Safety
When burning candles in jars, it’s important to be aware of potential fire hazards. As the candle burns down, the jar can get very hot. If the jar gets too hot, it can crack or shatter, spilling hot wax. This can cause fires if flammable materials are nearby.
To prevent this, it’s recommended to place candle jars on a heat-resistant surface, not too close to anything flammable. Keep the wicks trimmed to 1⁄4 inch to prevent overly large flames. Extinguish the candle once it gets within 1-2 inches of the bottom. Don’t burn a candle for more than 4 hours at a time. Allow the wax pool and jar to fully cool before touching or moving (NFPA).
Candle jars can explode if they get too hot, sending shattered glass around. Always keep candle jars out of reach of children and pets. Never leave a burning candle unattended. Make sure smoke detectors are installed and functional. Have a plan to carefully extinguish a candle if it gets out of control.
Conclusion
In summary, candles do evaporate when lit, as the heat melts and vaporizes the wax which allows it to evaporate. However, candles in jars can trap some of the evaporated wax, causing a build up on the sides of the glass. To prevent too much build up, it’s recommended to maintain the candle by cleaning the jar periodically. Properly lighting, placing and monitoring the candle can also help reduce excess evaporation and maintain the scent and ambiance. While candles in jars are generally safe, always take precautions against potential fire hazards. Overall, with some care and upkeep, candles in jars can provide enjoyable light and fragrance.