Does Candle Wax Just Evaporate?
The purpose of this article is to provide a comprehensive overview of what happens to candle wax when a candle burns. We will examine the composition of candle wax, the candle burning process, and where the wax goes when it evaporates. Understanding the science behind candle wax evaporation can help us extend the life of our candles and properly clean up any wax residue.
What is Candle Wax Made Of?
The primary component of most modern candle wax is paraffin, a petroleum-based wax. Paraffin wax is composed of saturated hydrocarbons with the chemical formula CnH2n+2, where n represents the number of carbon atoms.[1] It is a soft, colorless, tasteless solid derived from petroleum refining that melts at around 37–65°C.[2]
Other key ingredients in candle wax include:
- Stearic acid – a fatty acid that influences wax texture and burning characteristics
- Dyes and fragrances – for color and scent
- Vybar – improves flexibility and hardness
- Microcrystalline wax – increases opacity and rigidity
The specific proportions of these components vary between different candle wax types like paraffin, soy, beeswax, and gel. But paraffin remains the primary constituent of most commercial candle waxes today.
How Candles Burn
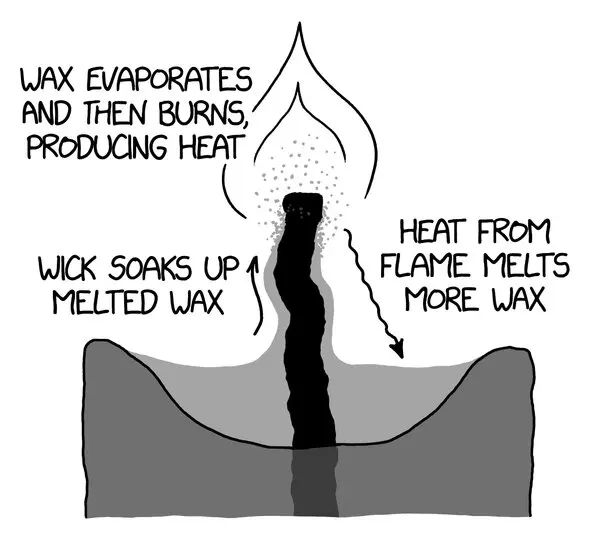
The key to understanding how candles burn involves looking at the role of the wick. As the candle burns, the wick acts like a fuel pump, drawing the liquefied wax up into the flame to allow it to vaporize and burn (Elements of a Candle: Wicks, https://candles.org/elements-of-a-candle/wicks/). The heat from the flame melts the solid wax into liquid form. This liquid wax is then transported up the wick through capillary action. The wick essentially acts as a sponge, soaking up the melted wax and bringing it closer to the flame. Once the liquid wax reaches the flame, it vaporizes into a gas and combusts.
This vaporization process turns the solid wax into a fuel for the flame. As the wax vaporizes, the flame consumes it and produces light, heat, water vapor, and carbon dioxide. Without the wick constantly pulling up more liquid wax, the flame would quickly run out of fuel and go out (How does a candle work? | HowStuffWorks – Home and Garden, https://home.howstuffworks.com/question267.htm). The wick sustains the burning process by delivering the melted wax to the flame through capillary action. So in summary, the wick is what allows the solid candle wax to be turned into a vapor fuel for the flame.
Factors Affecting Evaporation Rate
The rate at which candle wax evaporates depends on several factors including temperature, air currents, and the type of wax used in the candle.
Higher temperatures cause wax molecules to vibrate and move faster, increasing evaporation. According to one source, candles burn up to 20% faster at higher room temperatures (https://suffolkcandles.co.uk/blogs/candles/does-candle-wax-evaporate-when-it-is-burnt). Air currents from fans, open windows, or air conditioning also accelerate evaporation by carrying away wax vapors.
The type of wax impacts evaporation as well. Softer waxes like paraffin evaporate quicker than harder waxes like soy or beeswax, which have higher melting points. One source explains that soft wax candles can evaporate up to 50% faster than hard wax varieties (https://www.quora.com/Do-wax-melts-evaporate).
Understanding how these factors influence the evaporation rate can help determine optimal conditions for faster or slower burning.
Where Does the Wax Go?
When a candle burns, some of the wax undergoes a chemical reaction and turns into a gas through the process of vaporization (NYTimes). The wax vapors are released into the air and dissipate. However, not all of the wax gets vaporized. Some wax remains solid and can leave behind residue (ThoughtCo).
The chemical reaction that occurs when wax burns requires oxygen. The wax hydrocarbons combine with oxygen to produce carbon dioxide and water vapor. Most of the wax mass is lost as these gaseous products (NYTimes).
However, some solid wax remains unburned. This wax can melt and pool around the base of the candle. It can also splatter onto surfaces as the candle burns. Additionally, some soot may form from incomplete combustion. The soot contains unburned carbon particles from the wax. These deposits are left behind after the candle goes out (ThoughtCo).
So in summary, some of the candle wax vaporizes completely and some remains solid, either as splatter, pools of wax, or sooty residue. The vaporized portion enters the air as water vapor and carbon dioxide. But wax in solid form that doesn’t undergo the chemical reaction remains behind.
Soot Formation
Incomplete combustion of the candle wax can leave behind black carbon deposits known as soot. This soot is composed of unburned carbon particles that did not fully combust when the candle was lit (Source). Ideal combustion converts the wax hydrocarbon completely into carbon dioxide and water. However, insufficient oxygen supply, drafts, or disturbances to the flame can all lead to incomplete combustion and soot production.
The black soot can deposit on walls, furniture, ceilings, and other surfaces near the candle. Soot builds up over time with continued use. It starts out as a dusty, greasy film on surfaces before accumulating into more solid black stains if left unchecked. Preventing drafts, trimming wicks, and using candles properly will minimize soot, but some amount is inevitable from the combustion process itself (Source).
Wax Deposits
As a candle burns, some of the wax will liquefy from the heat of the flame. This melted wax can drip down onto surfaces near the candle, leaving behind wax deposits.
Candle wax is designed to be a solid at room temperature. However, the material can reach temperatures of 140 to 200°F next to the flame. At these high temperatures, the wax melts and becomes a viscous liquid that can drip or spill onto nearby surfaces (Source).
Common places where wax deposits form include candle holders, plates or trays under the candle, tabletops, and other surfaces near the flame. The closer the material is to the flame, the more severe the wax buildup will be.
Wax drippings tend to flow downward due to gravity. So wax deposits frequently accumulate on vertical surfaces directly below the flame, as the liquefied wax drips down. However, wax can splatter in any direction, particularly if disturbed while liquid.
The amount of wax that deposits also depends on the type of wax. Some waxes melt at lower temperatures and produce more liquid, while harder waxes melt less and drip less. Container candles tend to build up more exterior wax than taper or pillar candles.
Prolonged burning or breeze/drafts can exacerbate wax deposits by blowing the flame and consequently the melted wax. But with careful monitoring, wax buildup can be minimized.
Clean Up and Removal
It’s important to clean up any wax residue and drips after candles burn to avoid further staining or buildup. Here are some tips for removing wax from common surfaces:
For wood surfaces like furniture or floors, fill a plastic bag with ice cubes and place it on top of the wax deposit. Let the ice sit until the wax becomes brittle and starts to flake off, then gently scrape away any remaining wax with a plastic scraper or old credit card. Avoid using sharp metal tools that could further damage the wood. If any wax remains, try using a wax remover product designed for wood or an oil like olive or vegetable oil, rubbing it into the wood with a soft cloth and wiping away.
On glass like windows, mirrors or tabletops, allow the wax to fully harden then gently peel or roll it off starting from the edge. Any residue can be removed with glass cleaner or rubbing alcohol. For fabric or carpet, start by scraping off any thick chunks you can. Then place a paper towel or blotting cloth over the wax stain and apply a heated iron on medium heat to melt the wax into the paper. Switch out paper towels and repeat until no more wax transfers. For any remaining stain treat with a small amount of dish soap and water or use a carpet cleaner product.
Metal surfaces like candle holders can be placed in the freezer until the wax gets hard and brittle, then gently break off or scrape away any excess buildup with a plastic scraper. Rubbing alcohol, vinegar or grease-fighting dish soap can help remove any remaining residue. Avoid using extremely hot water to try to melt wax off surfaces, as this often spreads the wax further. With some patience and the right techniques, wax can be effectively removed from most surfaces.
Safety Considerations
Proper candle safety is crucial to prevent fires. According to the National Fire Protection Association (NFPA), candles cause an estimated 7,000 house fires each year, resulting in around 80 deaths annually (Source). Here are some key tips to burn candles safely:
Maintain the proper wick length – Clip the wick to 1⁄4” before lighting to avoid high flames. Long or crooked wicks can create more smoke and dripping.
Allow for adequate ventilation – Burn candles in an open room, avoiding drafts or air currents. Good airflow prevents rapid or uneven burning, sooting, and excessive dripping (Source).
Watch for fire hazards – Keep candles away from flammable items like curtains, books, trees, and decorations. Place on a sturdy, nonflammable surface away from edges. Never leave burning candles unattended.
Extinguish completely – Blow out candles when you leave a room or go to sleep. Pouring water on the melted wax can help ensure it is fully extinguished and no wick smoldering remains.
With proper precautions, you can safely enjoy the light, scent, and atmosphere candles create. But always practice fire safety first, as an open flame poses serious risks if not properly handled.
Conclusion
As we have discussed, candle wax is composed mainly of paraffin or beeswax which melts into a liquid when heated. As the candle burns, the melted wax vaporizes into the air through evaporation. However, some residual wax may condense as it cools and deposit on surfaces near the candle. Proximity to the flame and air circulation are key factors affecting the rate of wax evaporation. With proper ventilation and reasonable burn times, most of the wax will turn into vapor. Any remaining wax deposits can be cleaned with hot water, rubbing alcohol or commercial wax removers. Candles release small amounts of soot or unburned carbon from incomplete combustion. Proper wick trimming helps minimize soot. In summary, under normal conditions, most candle wax evaporates into the air as vapor when burned, with minimal deposits and residue.





